Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer
- PMID: 35306155
- PMCID: PMC9067454
- DOI: 10.1016/j.annonc.2022.02.007
Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer
Abstract
Background: Identification of residual disease in patients with localized non-small cell lung cancer (NSCLC) following treatment with curative intent holds promise to identify patients at risk of relapse. New methods can detect circulating tumour DNA (ctDNA) in plasma to fractional concentrations as low as a few parts per million, and clinical evidence is required to inform their use.
Patients and methods: We analyzed 363 serial plasma samples from 88 patients with early-stage NSCLC (48.9%/28.4%/22.7% at stage I/II/III), predominantly adenocarcinomas (62.5%), treated with curative intent by surgery (n = 61), surgery and adjuvant chemotherapy/radiotherapy (n = 8), or chemoradiotherapy (n = 19). Tumour exome sequencing identified somatic mutations and plasma was analyzed using patient-specific RaDaR™ assays with up to 48 amplicons targeting tumour-specific variants unique to each patient.
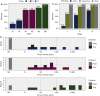
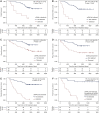
Results: ctDNA was detected before treatment in 24%, 77% and 87% of patients with stage I, II and III disease, respectively, and in 26% of all longitudinal samples. The median tumour fraction detected was 0.042%, with 63% of samples <0.1% and 36% of samples <0.01%. ctDNA detection had clinical specificity >98.5% and preceded clinical detection of recurrence of the primary tumour by a median of 212.5 days. ctDNA was detected after treatment in 18/28 (64.3%) of patients who had clinical recurrence of their primary tumour. Detection within the landmark timepoint 2 weeks to 4 months after treatment end occurred in 17% of patients, and was associated with shorter recurrence-free survival [hazard ratio (HR): 14.8, P <0.00001] and overall survival (HR: 5.48, P <0.0003). ctDNA was detected 1-3 days after surgery in 25% of patients yet was not associated with disease recurrence. Detection before treatment was associated with shorter overall survival and recurrence-free survival (HR: 2.97 and 3.14, P values 0.01 and 0.003, respectively).
Conclusions: ctDNA detection after initial treatment of patients with early-stage NSCLC using sensitive patient-specific assays has potential to identify patients who may benefit from further therapeutic intervention.
Keywords: cell-free DNA (cfDNA); circulating tumour DNA (ctDNA); early detection; liquid biopsy; minimal residual disease (MRD); non-small cell lung cancer (NSCLC).
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure NR and DGa are co-founders, and NR, DGa, MP, KHo, SH, GM, CGS and GS are current or former employees/officers/consultants of Inivata Ltd. Inivata provided analysis of samples using the RaDaR™ assays. DGa and KHe are current employees of AstraZeneca Inc. All other authors have declared no conflicts of interest.
Figures


References
-
- Globocan 2020. World Health Organisation, International Agency for Research on Cancer. https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf Available at. Accessed March 18, 2020.
-
- Cancer Research UK. 2020. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... Available at. Accessed March 18, 2020.
-
- NICE. Lung cancer in adults: treatments with curative intent. Webpage. 2021. https://www.nice.org.uk/guidance/qs17/chapter/Quality-statement-5-Treatm... Available at. Accessed March 18, 2020.
-
- Wan J.C.M., Massie C., Garcia-Corbacho J., et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–238. - PubMed
-
- Dawson S.-J., Tsui D.W.Y., Murtaza M., et al. Analysis of circulating tumour DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

