Live cell imaging of oxidative stress in human airway epithelial cells exposed to isoprene hydroxyhydroperoxide
- PMID: 35306372
- PMCID: PMC8933716
- DOI: 10.1016/j.redox.2022.102281
Live cell imaging of oxidative stress in human airway epithelial cells exposed to isoprene hydroxyhydroperoxide
Abstract
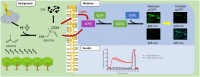
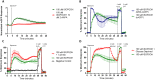
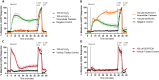
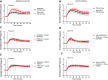
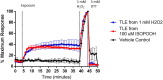
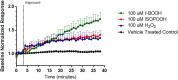
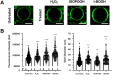
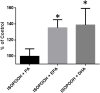
Exposure to respirable air particulate matter (PM2.5) in ambient air is associated with morbidity and premature deaths. A major source of PM2.5 is the photooxidation of volatile plant-produced organic compounds such as isoprene. Photochemical oxidation of isoprene leads to the formation of hydroperoxides, environmental oxidants that lead to inflammatory (IL-8) and adaptive (HMOX1) gene expression in human airway epithelial cells (HAEC). To examine the mechanism through which these oxidants alter intracellular redox balance, we used live-cell imaging to monitor the effects of isoprene hydroxyhydroperoxides (ISOPOOH) in HAEC expressing roGFP2, a sensor of the glutathione redox potential (EGSH). Non-cytotoxic exposure of HAEC to ISOPOOH resulted in a rapid and robust increase in EGSH that was independent of the generation of intracellular or extracellular hydrogen peroxide. Our results point to oxidation of GSH through the redox relay initiated by glutathione peroxidase 4, directly by ISOPOOH or indirectly by ISOPOOH-generated lipid hydroperoxides. We did not find evidence for involvement of peroxiredoxin 6. Supplementation of HAEC with polyunsaturated fatty acids enhanced ISOPOOH-induced glutathione oxidation, providing additional evidence that ISOPOOH initiates lipid peroxidation of cellular membranes. These findings demonstrate that ISOPOOH is a potent environmental airborne hydroperoxide with the potential to contribute to oxidative burden of human airway posed by inhalation of secondary organic aerosols.
Keywords: Air pollution; Glutathione redox potential; Lipid peroxidation; Live cell imagining; Oxidative stress; Secondary organic aerosols.
Published by Elsevier B.V.
Figures

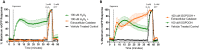

References
-
- Shrivastava M., Cappa C.D., Fan J., Goldstein A.H., Guenther A.B., Jimenez J.L., Kuang C., Laskin A., Martin S.T., Ng N.L., Petaja T., Pierce J.R., Rasch P.J., Roldin P., Seinfeld J.H., Shilling J., Smith J.N., Thornton J.A., Volkamer R., Wang J., Worsnop D.R., Zaveri R.A., Zelenyuk A., Zhang Q. Recent advances in understanding secondary organic aerosol: implications for global climate forcing. Rev. Geophys. 2017;55:509–559. doi: 10.1002/2016RG000540. - DOI
-
- Pandis S.N., Harley R.A., Cass G.R., Seinfeld J.H. Secondary organic aerosol formation and transport. Atmos. Environ. Part A, Gen. Top. 1992;26:2269–2282. doi: 10.1016/0960-1686(92)90358-R. - DOI
-
- Guenther A.B., Jiang X., Heald C.L., Sakulyanontvittaya T., Duhl T., Emmons L.K., Wang X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012;5:1471–1492. doi: 10.5194/gmd-5-1471-2012. - DOI
-
- Samet J.M., Zeger S.L., Dominici F., Curriero F., Coursac I., Dockery D.W. 2000. The National Morbidity , Mortality , Part II : Morbidity and Mortality from Air Pollution in the United States Final Version Includes a Commentary by the Institute ’ S Health Review Committee. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

