Neutrophil-Derived Myeloperoxidase and Hypochlorous Acid Critically Contribute to 20-Hydroxyeicosatetraenoic Acid Increases that Drive Postischemic Angiogenesis
- PMID: 35306474
- PMCID: PMC9190235
- DOI: 10.1124/jpet.121.001036
Neutrophil-Derived Myeloperoxidase and Hypochlorous Acid Critically Contribute to 20-Hydroxyeicosatetraenoic Acid Increases that Drive Postischemic Angiogenesis
Abstract
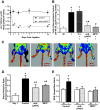
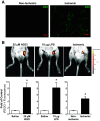
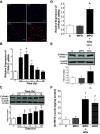
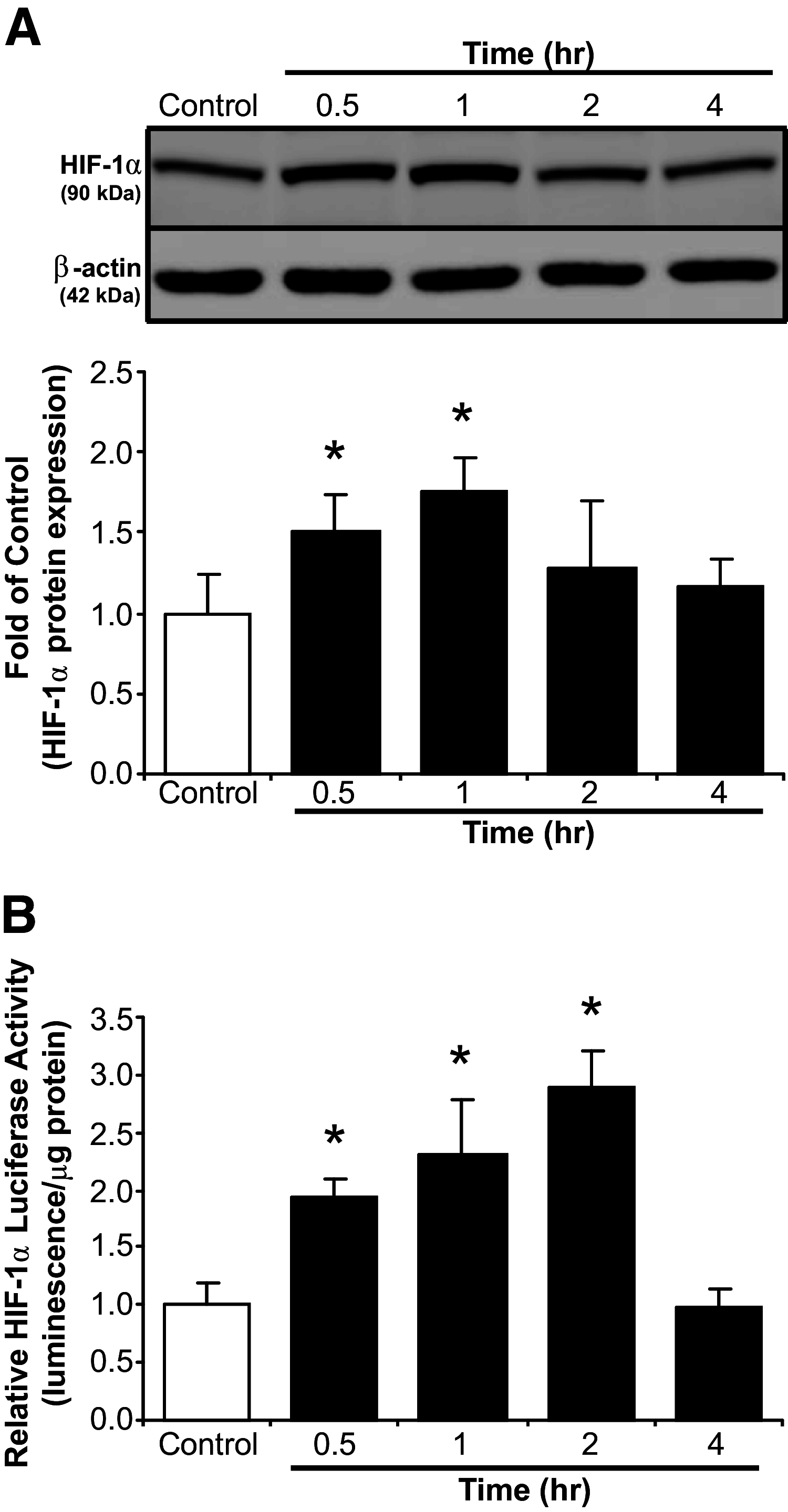
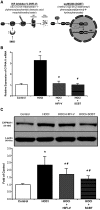
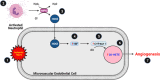
Compensatory angiogenesis is an important adaptation for recovery from critical ischemia. We recently identified 20-hydroxyeicosatetraenoic acid (20-HETE) as a novel contributor of ischemia-induced angiogenesis. However, the precise mechanisms by which ischemia promotes 20-HETE increases that drive angiogenesis are unknown. This study aims to address the hypothesis that inflammatory neutrophil-derived myeloperoxidase (MPO) and hypochlorous acid (HOCl) critically contribute to 20-HETE increases leading to ischemic angiogenesis. Using Liquid Chromatography-Mass Spectrometry/Mass Spectrometry, Laser Doppler Perfusion Imaging, and Microvascular Density analysis, we found that neutrophil depletion and MPO knockout mitigate angiogenesis and 20-HETE production in the gracilis muscles of mice subjected to hindlimb ischemia. Furthermore, we found MPO and HOCl to be elevated in these tissues postischemia as assessed by immunofluorescence microscopy and in vivo live imaging of HOCl. Next, we demonstrated that the additions of either HOCl or an enzymatic system for generating HOCl to endothelial cells increase the expression of CYP4A11 and its product, 20-HETE. Finally, pharmacological interference of hypoxia inducible factor (HIF) signaling results in ablation of HOCl-induced CYP4A11 transcript and significant reductions in CYP4A11 protein. Collectively, we conclude that neutrophil-derived MPO and its product HOCl activate HIF-1α and CYP4A11 leading to increased 20-HETE production that drives postischemic compensatory angiogenesis. SIGNIFICANCE STATEMENT: Traditionally, neutrophil derived MPO and HOCl are exclusively associated in the innate immunity as potent bactericidal/virucidal factors. The present study establishes a novel paradigm by proposing a unique function for MPO/HOCl as signaling agents that drive critical physiological angiogenesis by activating the CYP4A11-20-HETE signaling axis via a HIF-1α-dependent mechanism. The findings from this study potentially identify novel therapeutic targets for the treatment of ischemia and other diseases associated with abnormal angiogenesis.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Antonelou M, Michaëlsson E, Evans RDR, Wang CJ, Henderson SR, Walker LSK, Unwin RJ, Salama AD; RAVE-ITN Investigators (2020) Therapeutic myeloperoxidase inhibition attenuates neutrophil activation, anca-mediated endothelial damage, and crescentic GN. J Am Soc Nephrol 31:350–364 10.1681/ASN.2019060618. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

