An anti-ANGPTL3/8 antibody decreases circulating triglycerides by binding to a LPL-inhibitory leucine zipper-like motif
- PMID: 35307397
- PMCID: PMC9036128
- DOI: 10.1016/j.jlr.2022.100198
An anti-ANGPTL3/8 antibody decreases circulating triglycerides by binding to a LPL-inhibitory leucine zipper-like motif
Abstract
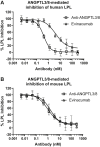
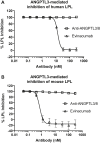
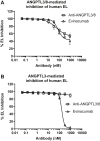
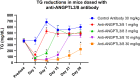
Triglycerides (TG) are required for fatty acid transport and storage and are essential for human health. Angiopoietin-like-protein 8 (ANGPTL8) has previously been shown to form a complex with ANGPTL3 that increases circulating TG by potently inhibiting LPL. We also recently showed that the TG-lowering apolipoprotein A5 (ApoA5) decreases TG levels by suppressing ANGPTL3/8-mediated LPL inhibition. To understand how LPL binds ANGPTL3/8 and ApoA5 blocks this interaction, we used hydrogen-deuterium exchange mass-spectrometry and molecular modeling to map binding sites of LPL and ApoA5 on ANGPTL3/8. Remarkably, we found that LPL and ApoA5 both bound a unique ANGPTL3/8 epitope consisting of N-terminal regions of ANGPTL3 and ANGPTL8 that are unmasked upon formation of the ANGPTL3/8 complex. We further used ANGPTL3/8 as an immunogen to develop an antibody targeting this same epitope. After refocusing on antibodies that bound ANGPTL3/8, as opposed to ANGPTL3 or ANGPTL8 alone, we utilized bio-layer interferometry to select an antibody exhibiting high-affinity binding to the desired epitope. We revealed an ANGPTL3/8 leucine zipper-like motif within the anti-ANGPTL3/8 epitope, the LPL-inhibitory region, and the ApoA5-interacting region, suggesting the mechanism by which ApoA5 lowers TG is via competition with LPL for the same ANGPTL3/8-binding site. Supporting this hypothesis, we demonstrate that the anti-ANGPTL3/8 antibody potently blocked ANGPTL3/8-mediated LPL inhibition in vitro and dramatically lowered TG levels in vivo. Together, these data show that an anti-ANGPTL3/8 antibody targeting the same leucine zipper-containing epitope recognized by LPL and ApoA5 markedly decreases TG by suppressing ANGPTL3/8-mediated LPL inhibition.
Keywords: angiopoietin-like protein (ANGPTL); apolipoprotein (Apo); epitopes; hydrogen-deuterium exchange mass spectrometry (HDXMS); leucine zipper; lipoprotein lipase (LPL); molecular modeling; nano-imaging; transmission electron microscopy (TEM); triglycerides (TG).
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Kersten S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta. 2014;1841:919–933. - PubMed
-
- He P.P., Jiang T., OuYang X.P., Liang Y.Q., Zou J.Q., Wang Y., Shen Q.Q., Liao L., Zheng X.L. Lipoprotein lipase: biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clin. Chim. Acta. 2018;480:126–137. - PubMed
-
- Beigneux A.P., Allan C.M., Sandoval N.P., Cho G.W., Heizer P.J., Jung R.S., Stanhope K.L., Havel P.J., Birrane G., Meiyappan M., Gill J.E.t., Murakami M., Miyashita K., Nakajima K., Ploug M., et al. Lipoprotein lipase is active as a monomer. Proc. Natl. Acad. Sci. U. S. A. 2019;116:6319–6328. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

