Hepatic stellate cells in physiology and pathology
- PMID: 35307840
- PMCID: PMC9012702
- DOI: 10.1113/JP281061
Hepatic stellate cells in physiology and pathology
Abstract
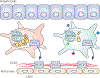
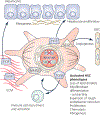
Hepatic stellate cells (HSCs) comprise a minor cell population in the liver but serve numerous critical functions in the normal liver and in response to injury. HSCs are primarily known for their activation upon liver injury and for producing the collagen-rich extracellular matrix in liver fibrosis. In the absence of liver injury, HSCs reside in a quiescent state, in which their main function appears to be the storage of retinoids or vitamin A-containing metabolites. Less appreciated functions of HSCs include amplifying the hepatic inflammatory response and expressing growth factors that are critical for liver development and both the initiation and termination of liver regeneration. Recent single-cell RNA sequencing studies have corroborated earlier studies indictaing that HSC activation involves a diverse array of phenotypic alterations and identified unique HSC populations. This review serves to highlight these many functions of HSCs, and to briefly describe the recent genetic tools that will help to thoroughly investigate the role of HSCs in hepatic physiology and pathology.
Keywords: fibrosis; liver; stellate cell.
© 2022 The Authors. The Journal of Physiology © 2022 The Physiological Society.
Conflict of interest statement
Competing interests
The authors have no conflicts of interest to declare.
Figures

References
-
- Andrews TS, Atif J, Liu JC, Perciani CT, Ma XZ, Thoeni C, Slyper M, Eraslan G, Segerstolpe A, Manuel J, Chung S, Winter E, Cirlan I, Khuu N, Fischer S, Rozenblatt-Rosen O, Regev A, McGilvray ID, Bader GD & MacParland SA. (2021). Single-Cell, Single-Nucleus, and Spatial RNA Sequencing of the Human Liver Identifies Cholangiocyte and Mesenchymal Heterogeneity. Hepatol Commun. - PMC - PubMed
-
- Baba S, Fujii H, Hirose T, Yasuchika K, Azuma H, Hoppo T, Naito M, Machimoto T & Ikai I. (2004). Commitment of bone marrow cells to hepatic stellate cells in mouse. J Hepatol 40, 255–260. - PubMed
-
- Ballardini G, Groff P, Badiali de Giorgi L, Schuppan D & Bianchi FB. (1994). Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology 19, 440–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

