A physiologically based pharmacokinetic model of clopidogrel in populations of European and Japanese ancestry: An evaluation of CYP2C19 activity
- PMID: 35307978
- PMCID: PMC8934724
- DOI: 10.1002/prp2.946
A physiologically based pharmacokinetic model of clopidogrel in populations of European and Japanese ancestry: An evaluation of CYP2C19 activity
Abstract
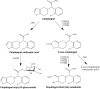
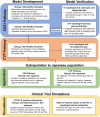
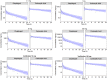
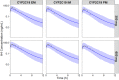
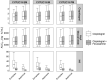
Treatment response to clopidogrel is associated with CYP2C19 activity through the formation of the active H4 metabolite. The aims of this study were to develop a physiologically based pharmacokinetic (PBPK) model of clopidogrel and its metabolites for populations of European ancestry, to predict the pharmacokinetics in the Japanese population by CYP2C19 phenotype, and to investigate the effect of clinical and demographic factors. A PBPK model was developed and verified to describe the two metabolic pathways of clopidogrel (H4 metabolite, acyl glucuronide metabolite) for a population of European ancestry using plasma data from published studies. Subsequently, model predictions in the Japanese population were evaluated. The effects of CYP2C19 activity, fluvoxamine coadministration (CYP2C19 inhibitor), and population-specific factors (age, sex, BMI, body weight, cancer, hepatic, and renal dysfunction) on the pharmacokinetics of clopidogrel and its metabolites were then characterized. The predicted/observed ratios for clopidogrel and metabolite exposure parameters were acceptable (twofold acceptance criteria). For all CYP2C19 phenotypes, steady-state AUC0-τ of the H4 metabolite was lower for the Japanese (e.g., EM, 7.69 [6.26-9.45] ng·h/ml; geometric mean [95% CI]) than European (EM, 24.8 [20.4-30.1] ng·h/ml, p < .001) population. In addition to CYP2C19-poor metabolizer phenotype, fluvoxamine coadministration, hepatic, and renal dysfunction were found to reduce H4 metabolite but not acyl glucuronide metabolite concentrations. This is the first PBPK model describing the two major metabolic pathways of clopidogrel, which can be applied to populations of European and Japanese ancestry by CYP2C19 phenotype. The differences between the two populations appear to be determined primarily by the effect of varying CYP2C19 liver activity.
Keywords: CYP2C19; PBPK modelling; clopidogrel; inter-ethnic differences; metabolites.
© 2022 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
All authors declare no conflicts of interest that could have influenced the submitted work.
Figures



References
-
- Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417‐423. - PubMed
-
- Dorji PW, Tshering G, Na‐Bangchang K. CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South‐East and East Asian populations: a systematic review. J Clin Pharm Ther. 2019;44:508‐524. - PubMed
-
- Feng S, Cleary Y, Parrott N, et al. Evaluating a physiologically based pharmacokinetic model for prediction of omeprazole clearance and assessing ethnic sensitivity in CYP2C19 metabolic pathway. Eur J Clin Pharmacol. 2015;71:617‐624. - PubMed
-
- Sim SC, Risinger C, Dahl ML, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103‐113. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

