Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What is the Evidence so far?
- PMID: 35308198
- PMCID: PMC8924552
- DOI: 10.3389/fphar.2022.838782
Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What is the Evidence so far?
Abstract
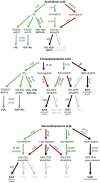
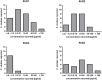
Formation of specialized pro-resolving lipid mediators (SPMs) such as lipoxins or resolvins usually involves arachidonic acid 5-lipoxygenase (5-LO, ALOX5) and different types of arachidonic acid 12- and 15-lipoxygenating paralogues (15-LO1, ALOX15; 15-LO2, ALOX15B; 12-LO, ALOX12). Typically, SPMs are thought to be formed via consecutive steps of oxidation of polyenoic fatty acids such as arachidonic acid, eicosapentaenoic acid or docosahexaenoic acid. One hallmark of SPM formation is that reported levels of these lipid mediators are much lower than typical pro-inflammatory mediators including the monohydroxylated fatty acid derivatives (e.g., 5-HETE), leukotrienes or certain cyclooxygenase-derived prostaglandins. Thus, reliable detection and quantification of these metabolites is challenging. This paper is aimed at critically evaluating i) the proposed biosynthetic pathways of SPM formation, ii) the current knowledge on SPM receptors and their signaling cascades and iii) the analytical methods used to quantify these pro-resolving mediators in the context of their instability and their low concentrations. Based on current literature it can be concluded that i) there is at most, a low biosynthetic capacity for SPMs in human leukocytes. ii) The identity and the signaling of the proposed G-protein-coupled SPM receptors have not been supported by studies in knock-out mice and remain to be validated. iii) In humans, SPM levels were neither related to dietary supplementation with their ω-3 polyunsaturated fatty acid precursors nor were they formed during the resolution phase of an evoked inflammatory response. iv) The reported low SPM levels cannot be reliably quantified by means of the most commonly reported methodology. Overall, these questions regarding formation, signaling and occurrence of SPMs challenge their role as endogenous mediators of the resolution of inflammation.
Keywords: FPR; LC-MS-based lipid mediator analysis; SPM; leukotriene; lipoxin; lipoxygenase; resolution of inflammation; resolvin.
Copyright © 2022 Schebb, Kühn, Kahnt, Rund, O’Donnell, Flamand, Peters-Golden, Jakobsson, Weylandt, Rohwer, Murphy, Geisslinger, FitzGerald, Hanson, Dahlgren, Alnouri, Offermanns and Steinhilber.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Allen-Redpath K., Aldrovandi M., Lauder S. N., Gketsopoulou A., Tyrrell V. J., Slatter D. A., et al. (2019). Phospholipid Membranes Drive Abdominal Aortic Aneurysm Development through Stimulating Coagulation Factor Activity. Proc. Natl. Acad. Sci. U S A. 116, 8038–8047. 10.1073/pnas.1814409116 - DOI - PMC - PubMed
-
- Archambault A. S., Brassard J., Bernatchez É., Martin C., Di Marzo V., Laviolette M., et al. (2022). Human and Mouse Eosinophils Differ in Their Ability to Biosynthesize Eicosanoids, Docosanoids, the Endocannabinoid 2-Arachidonoyl-Glycerol and its Congeners. Cells 11, 141. 10.3390/cells11010141 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

