Effectiveness and Safety Analysis of PIs/r Based Dual Therapy in Treatment-Naïve, HIV/AIDS Patients: A Network Meta Analysis of Randomized Controlled Trials
- PMID: 35308227
- PMCID: PMC8931831
- DOI: 10.3389/fphar.2022.811357
Effectiveness and Safety Analysis of PIs/r Based Dual Therapy in Treatment-Naïve, HIV/AIDS Patients: A Network Meta Analysis of Randomized Controlled Trials
Abstract
Background: Dual anti-retroviral therapy is the main proven valuable intervention type for treating naïve HIV/AIDS. Currently, no high-quality evidence is available regarding the best dual schemes. Objectives: The aim of this study is to evaluate the effectiveness and safety of PIs/r-based dual therapy in treatment-naïve HIV/AIDS patients by using network meta-analysis. Methods: Randomized controlled trials of PIs/r-based dual therapy in treatment-naïve HIV/AIDS were searched based on Embase, PubMed and Cochrane library database from January 2006 to June 2021. Taking viral suppression rate, CD4+T cell count changes from baseline as the primary indicator and adverse events rate as secondary indicator, the network meta-analysis was performed on Review Manager and STATA software. Heterogeneity was assessed by the Q statistic and I2. We registered our protocol in Prospero with ID CRD42021275466. Results: Among 15 randomized controlled trials (3,497 patients and 7 PIs/r-based dual therapy) were reviewed in this study. According to the forest map, DRV/r + INSTIs was more effective compared to triple therapy (TT) in viral suppression [OR 0.82, 95% CI (0.61-1.11)], in CD4+T cell count changes from baseline [MD 1.9, 95% CI (0.7, 3.1), I 2 86%], in adverse events [OR 0.98, 95% CI (0.68-1.39)]. Furthermore, SUCRA ranking analysis indicated that DRV/r + INSTIs was superior to TT in viral suppression (DRV/r + INSTIs 75.5% > TT 41.2%) and in immune construction (DRV/r + INSTIs 67% > TT 42%). In addition, DRV/r + INSTIs was similar to TT in adverse events (DRV/r + INSTIs 54.9% ≈ TT 54.7%). Conclusion: DRV/r + INSTIs was obviously superior to TT in viral suppression and immune reconstruction, and was not higher than TT in adverse events. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021275466.
Keywords: HIV infection; PIs/r; aids; dual therapy; network meta-analysis; triple therapy.
Copyright © 2022 Hui, Xiaoxu, Yuqi, Peng, Xin, Yunyun and Xin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

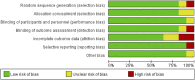


References
-
- Batchelder A. W., Brisbane M., Litwin A. H., Nahvi S., Berg K. M., Arnsten J. H. (2013). "Damaging what Wasn't Damaged Already": Psychological Tension and Antiretroviral Adherence Among HIV-Infected Methadone-Maintained Drug Users. AIDS Care 25 (11), 1370–1374. 10.1080/09540121.2013.766303 - DOI - PMC - PubMed
-
- Bedimo R. J., Drechsler H., Jain M., Cutrell J., Zhang S., Li X., et al. (2014). The RADAR Study: Week 48 Safety and Efficacy of Raltegravir Combined with Boosted Darunavir Compared to Tenofovir/emtricitabine Combined with Boosted Darunavir in Antiretroviral-Naive Patients. Impact on Bone Health. PloS one 9 (8), e106221. 10.1371/journal.pone.0106221 - DOI - PMC - PubMed
-
- Buti J., Glenny A. M., Worthington H. V., Nieri M., Baccini M. (2011). Network Meta-Analysis of Randomised Controlled Trials: Direct and Indirect Treatment Comparisons. Eur. J. Oral Implantol. 4 (1), 55–62. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

