High Affinity Iron Acquisition Systems Facilitate but Are Not Essential for Colonization of Chickens by Salmonella Enteritidis
- PMID: 35308377
- PMCID: PMC8928163
- DOI: 10.3389/fmicb.2022.824052
High Affinity Iron Acquisition Systems Facilitate but Are Not Essential for Colonization of Chickens by Salmonella Enteritidis
Abstract
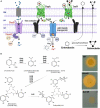
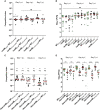
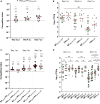
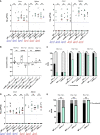
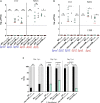
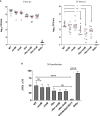
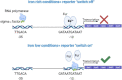
The roles of TonB mediated Fe3+ (ferric iron) uptake via enterobactin (involving biosynthesis genes entABCDEF) and Fe2+ (ferrous iron) uptake through the FeoABC transporter are poorly defined in the context of chicken-Salmonella interactions. Both uptake systems are believed to be the major contributors of iron supply in the Salmonella life cycle. Current evidence suggests that these iron uptake systems play a major role in pathogenesis in mammals and as such, they represent promising antibacterial targets with therapeutic potential. We investigated the role of these iron uptake mechanisms regarding the ability of Salmonella Enteritidis (SEn) strains to colonize in a chicken infection model. Further we constructed a bioluminescent reporter to sense iron limitation during gastrointestinal colonization of Salmonella in chicken via ex vivo imaging. Our data indicated that there is some redundancy between the ferric and ferrous iron uptake mechanisms regarding iron acquisition during SEn pathogenesis in chicken. We believe that this redundancy of iron acquisition in the host reservoir may be the consequence of adaptation to unique avian environments, and thus warrants further investigation. To our knowledge, this the first report providing direct evidence that both enterobactin synthesis and FeoABC mediated iron uptake contribute to the virulence of SEn in chickens.
Keywords: FeoABC transporter; Salmonella; chicken; enterobactin; iron uptake.
Copyright © 2022 Wellawa, Lam, White, Gomis, Allan and Köster.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Andrews S. C., Robinson A. K., Rodríguez-Quiñones F. (2003). Bacterial iron homeostasis. FEMS Microbiol. Rev. 27 215–237. - PubMed
-
- Bailey J., Cox N., Cosby D., Richardson L. (2005). Movement and persistence of Salmonella in broiler chickens following oral or intracloacal inoculation. J. Food Prot. 68 2698–2701. - PubMed
-
- Barrow P. (1991). Experimental infection of chickens with Salmonella enteritidis. Avian Pathol. 20 145–153. - PubMed
-
- Befus A. D., Johnston N., Leslie G., Bienenstock J. (1980). Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer’s patches. J. Immunol. 125 2626–2632. - PubMed
LinkOut - more resources
Full Text Sources

