A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery
- PMID: 35308783
- PMCID: PMC8924030
- DOI: 10.1016/j.omtm.2022.03.009
A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery
Abstract
Current RNA vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are limited by instability of both the RNA and the lipid nanoparticle delivery system, requiring storage at -20°C or -70°C and compromising universally accessible vaccine distribution. This study demonstrates the thermostability and adaptability of a nanostructured lipid carrier (NLC) delivery system for RNA vaccines that has the potential to address these concerns. Liquid NLC alone is stable at refrigerated temperatures for ≥1 year, enabling stockpiling and rapid deployment by point-of-care mixing with any vaccine RNA. Alternatively, NLC complexed with RNA may be readily lyophilized and stored at room temperature for ≥8 months or refrigerated temperature for ≥21 months while still retaining the ability to express protein in vivo. The thermostability of this NLC/RNA vaccine delivery platform could significantly improve distribution of current and future pandemic response vaccines, particularly in low-resource settings.
Keywords: RNA vaccine; RNA vaccine platform; lyophilization; nanostructured lipid carrier (NLC); saRNA; stability; thermostability.
© 2022 The Authors.
Conflict of interest statement
C.F. and N.V.H. are co-inventors on patent applications relating to PCT/US2018/37,783, “Nanostructured lipid carriers and stable emulsions and uses thereof.” M.A., A.G., E.V., and R.K. are co-inventors on US patent application nos. PCT/US21/40,388; 63/075,032; and 63/107,383, “Co-lyophilized RNA and nanostructured lipid carrier” and 63/144,169, “A thermostable, flexible RNA vaccine delivery platform for pandemic response.” All other authors declare no competing interests.
Figures
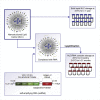
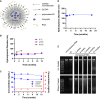
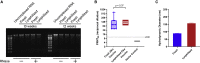

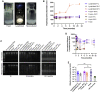
References
-
- Deering R.P., Kommareddy S., Ulmer J.B., Brito L.A., Geall A.J. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 2014;11:885–899. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

