Stereoretentive and regioselective selenium-catalyzed intermolecular propargylic C-H amination of alkynes
- PMID: 35308840
- PMCID: PMC8849008
- DOI: 10.1039/d1sc07067c
Stereoretentive and regioselective selenium-catalyzed intermolecular propargylic C-H amination of alkynes
Abstract
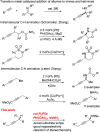
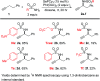
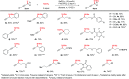
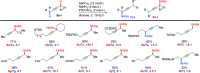
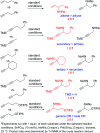
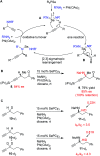
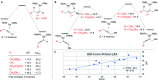
Herein we report an intermolecular propargylic C-H amination of alkynes. This reaction is operationally convenient and requires no transition metal catalysts or additives. Terminal, silyl, and internal alkynes bearing a wide range of functional groups can be aminated in high yields. The regioselectivity of amination for unsymmetrical internal alkynes is strongly influenced by substitution pattern (tertiary > secondary > primary) and by relatively remote heteroatomic substituents. We demonstrate that amination of alkynes bearing α-stereocenters occurs with retention of configuration at the newly-formed C-N bond. Competition experiments between alkynes, kinetic isotope effects, and DFT calculations are performed to confirm the mechanistic hypothesis that initial ene reaction of a selenium bis(imide) species is the rate- and product-determining step. This ene reaction has a transition state that results in substantial partial positive charge development at the carbon atom closer to the amination position. Inductive and/or hyperconjugative stabilization or destabilization of this positive charge explains the observed regioselectivities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Boryl-Dictated Site-Selective Intermolecular Allylic and Propargylic C-H Amination.J Am Chem Soc. 2022 Aug 10;144(31):14380-14387. doi: 10.1021/jacs.2c06117. Epub 2022 Jul 27. J Am Chem Soc. 2022. PMID: 35895901
-
Harnessing the β-boron effect for regioselective Ru-catalyzed hydrosilylation of internal alkynes.Nat Commun. 2025 May 14;16(1):4469. doi: 10.1038/s41467-025-59823-x. Nat Commun. 2025. PMID: 40368983 Free PMC article.
-
Copper-catalyzed enantioselective propargylic amination of propargylic esters with amines: copper-allenylidene complexes as key intermediates.J Am Chem Soc. 2010 Aug 4;132(30):10592-608. doi: 10.1021/ja1047494. J Am Chem Soc. 2010. PMID: 20617844
-
Regioselective coupling of pentafluorophenyl substituted alkynes: mechanistic insight into the zirconocene coupling of alkynes and a facile route to conjugated polymers bearing electron-withdrawing pentafluorophenyl substituents.J Am Chem Soc. 2003 Apr 9;125(14):4199-211. doi: 10.1021/ja0209161. J Am Chem Soc. 2003. PMID: 12670242
-
Regioselective Anti-Silyllithiation of Propargylic Alcohols.J Org Chem. 2024 Mar 15;89(6):3677-3683. doi: 10.1021/acs.joc.2c01795. Epub 2022 Nov 7. J Org Chem. 2024. PMID: 36342367 Review.
Cited by
-
Transition Metal Mimetic π-Activation by Cationic Bismuth(III) Catalysts for Allylic C-H Functionalization of Olefins Using C═O and C═N Electrophiles.J Am Chem Soc. 2024 Aug 14;146(32):22122-22128. doi: 10.1021/jacs.4c06235. Epub 2024 Aug 5. J Am Chem Soc. 2024. PMID: 39102739 Free PMC article.
-
Diastereoconvergent synthesis of anti-1,2-amino alcohols with N-containing quaternary stereocenters via selenium-catalyzed intermolecular C-H amination.Chem Sci. 2022 Aug 3;13(33):9685-9692. doi: 10.1039/d2sc02648a. eCollection 2022 Aug 24. Chem Sci. 2022. PMID: 36091896 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

