Photocytotoxicity and photoinduced phosphine ligand exchange in a Ru(ii) polypyridyl complex
- PMID: 35308843
- PMCID: PMC8848995
- DOI: 10.1039/d1sc05647f
Photocytotoxicity and photoinduced phosphine ligand exchange in a Ru(ii) polypyridyl complex
Abstract
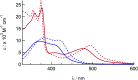
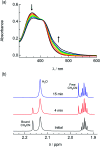
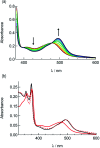
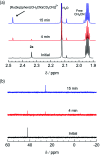
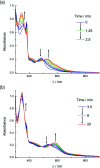
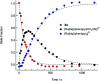
Two new tris-heteroleptic Ru(ii) complexes with triphenylphosphine (PPh3) coordination, cis-[Ru(phen)2(PPh3)(CH3CN)]2+ (1a, phen = 1,10-phenanthroline) and cis-[Ru(biq)(phen)(PPh3)(CH3CN)]2+ (2a, biq = 2,2'-biquinoline), were synthesized and characterized for photochemotherapeutic applications. Upon absorption of visible light, 1a exchanges a CH3CN ligand for a solvent water molecule. Surprisingly, the steady-state irradiation of 2a followed by electronic absorption and NMR spectroscopies reveals the photosubstitution of the PPh3 ligand. Phosphine photoinduced ligand exchange with visible light from a Ru(ii) polypyridyl complex has not previously been reported, and calculations reveal that it results from a trans-type influence in the excited state. Complexes 1a and 2a are not toxic against the triple negative breast cancer cell line MDA-MB-231 in the dark, but upon irradiation with blue light, the activity of both complexes increases by factors of >4.2 and 5.8, respectively. Experiments with PPh3 alone show that the phototoxicity observed for 2a does not arise from the released phosphine ligand, indicating the role of the photochemically generated ruthenium aqua complex on the biological activity. These complexes represent a new design motif for the selective release of PPh3 and CH3CN for use in photochemotherapy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Vittardi S. B. Magar R. T. Breen D. J. Rack J. J. J. Am. Chem. Soc. 2021;143:526–537. doi: 10.1021/jacs.0c08820. - DOI - PubMed
- King A. W. Wang L. Rack J. J. Acc. Chem. Res. 2015;48:1115–1122. doi: 10.1021/ar500396a. - DOI - PubMed
- McClure B. A. Mockus N. V. Butcher, Jr D. P. Lutterman D. A. Turro C. Petersen J. L. Rack J. J. Inorg. Chem. 2009;48:8084–8091. doi: 10.1021/ic900421v. - DOI - PubMed
-
- Karges J. Kuang S. Maschietto F. Blacque O. Ciofini I. Chao H. Gasser G. Nat. Commun. 2020;11:1–13. - PMC - PubMed
- Jakubaszek M. Goud B. Ferrari S. Gasser G. Chem. Commun. 2018;54:13040–13059. doi: 10.1039/C8CC05928D. - DOI - PubMed
- Mari C. Pierroz V. Ferrari S. Gasser G. Chem. Sci. 2015;6:2660–2686. doi: 10.1039/C4SC03759F. - DOI - PMC - PubMed
-
- White J. K. Schmehl R. H. Turro C. Inorg. Chim. Acta. 2017;454:7–20. doi: 10.1016/j.ica.2016.06.007. - DOI - PMC - PubMed
- Knoll J. D. Albani B. A. Turro C. Acc. Chem. Res. 2015;48:2280–2287. doi: 10.1021/acs.accounts.5b00227. - DOI - PMC - PubMed
- Knoll J. D. Turro C. Coord. Chem. Rev. 2015;282–283:110–126. doi: 10.1016/j.ccr.2014.05.018. - DOI - PMC - PubMed
- Sun Y. El Ojaimi M. Hammit R. Thummel R. P. Turro C. J. Phys. Chem. B. 2010;114:14664–14670. doi: 10.1021/jp102613n. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

