Effect of fingolimod on health-related quality of life in paediatric patients with multiple sclerosis: results from the phase 3 PARADIG MS Study
- PMID: 35308898
- PMCID: PMC8883212
- DOI: 10.1136/bmjno-2021-000215
Effect of fingolimod on health-related quality of life in paediatric patients with multiple sclerosis: results from the phase 3 PARADIG MS Study
Abstract
Background: In the PARADIGMS Study, fingolimod demonstrated superior efficacy versus interferon (IFN) β-1a and comparable overall incidence of adverse events but slightly higher rate of serious adverse events in patients with paediatric-onset multiple sclerosis (PoMS). Here, we report the health-related quality of life (HRQoL) outcomes from PARADIGMS.
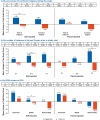
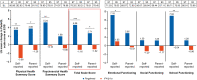
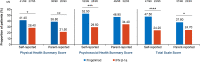
Methods: Patients with PoMS (N=215; aged 10-<18 years) were randomised to once-daily oral fingolimod (N=107) or once-weekly intramuscular IFN β-1a (N=108). HRQoL outcomes were assessed using the 23-item Pediatric Quality of Life (PedsQL) scale that comprises Physical and Psychosocial Health Summary Scores (including Emotional, Social and School Functioning). A post hoc inferential analysis evaluated changes in self-reported or parent-reported PedsQL scores from baseline up to 2 years between treatment groups using an analysis of covariance model.
Results: Treatment with fingolimod showed improvements versus IFN β-1a on the PedsQL scale in both the self-reported and parent-reported Total Scale Scores (4.66 vs -1.16, p≤0.001 and 2.71 vs -1.02, p≤0.05, respectively). The proportion of patients achieving a clinically meaningful improvement in the PedsQL Total Scale Score was two times higher with fingolimod versus IFN β-1a per the self-reported scores (47.5% vs 24.2%, p=0.001), and fingolimod was favoured versus IFN β-1a per the parent-reported scores (37.8% vs 24.7%, p=non-significant). Group differences in self-reported Total Scale Scores in favour of fingolimod were most pronounced among patients who had ≥2 relapses in the year prior to study entry or who showed improving or stable Expanded Disability Status Scale scores during the study.
Conclusion: Fingolimod improved HRQoL compared with IFN β-1a in patients with PoMS as evidenced by the self-reported and parent-reported PedsQL scores.
Keywords: multiple sclerosis; paediatric neurology; quality of life.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LK received personal compensation for activities as a consultant and/or participant on advisory boards for Biogen, Eisai, Gerson Lehrman, Janssen, Medscape, NeuroLive, Novartis, Roche and Sanofi. She has received royalties for the Fatigue Severity Scale from pharmaceutical and biotechnology companies, grant support from the National Multiple Sclerosis Society, and Department of Defense and research support from Novartis, Biogen, Genentech, the Lourie Foundation. She was compensated for her role as a back-up central MRI reviewer for the PARADIGMS Study. BB served as a consultant for Biogen Idec, Novartis, Teva Neuroscience, Merck Serono, Canadian MS Society Scientific Research Foundation, Canadian Multiple Sclerosis Society, National Multiple Sclerosis Society and Canadian Institutes of Health Research. She served as a remunerated central MRI reviewer for the present study. TC received personal compensation for advisory boards/consulting from F Hoffman-La Roche, Biogen and Novartis and financial support for research activities from the National Multiple Sclerosis Society, NIH and Department of Defense, Biogen, Merck Serono, Verily and Novartis. KD received personal compensation for speaker activities from Novartis, Servier, Biogen and Sanofi. JG, in the last 3 years, received honoraria for lectures and consultancy fees from Bayer, Novartis and Sanofi as well as funding for a research project from Novartis. AG received honoraria for speaking from Almirall, Biogen Idec, Merck Serono, Novartis, Genzyme and Sanofi-Aventis and for consultancy from Merck Serono, Biogen Idec, Teva, F Hoffmann-La Roche and Novartis. PH received honoraria for lectures and consultancy fees from Bayer, Merck, Biogen and Novartis. EW is funded by the NIH, NMSS, PCORI and Race to Erase MS. She volunteers on an advisory board for a Novartis trial. She is a site PI for clinical trials with Roche and Novartis. She has received honoraria from MS@TheLimit and The Corpus for educational talks. VD, AA and RK are employees of Novartis.
Figures


References
-
- The multiple sclerosis International Federation atlas of MS, 3rd ED, September, 2020. Available: https://www.Atlasofms.Org [Accessed 15 Nov 2021].
LinkOut - more resources
Full Text Sources
