Increased Prostaglandin E2 in Brainstem Respiratory Centers Is Associated With Inhibition of Breathing Movements in Fetal Sheep Exposed to Progressive Systemic Inflammation
- PMID: 35309054
- PMCID: PMC8928579
- DOI: 10.3389/fphys.2022.841229
Increased Prostaglandin E2 in Brainstem Respiratory Centers Is Associated With Inhibition of Breathing Movements in Fetal Sheep Exposed to Progressive Systemic Inflammation
Abstract
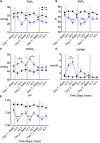
Background: Preterm newborns commonly experience apnoeas after birth and require respiratory stimulants and support. Antenatal inflammation is a common antecedent of preterm birth and inflammatory mediators, particularly prostaglandin E2 (PGE2), are associated with inhibition of vital brainstem respiratory centers. In this study, we tested the hypothesis that exposure to antenatal inflammation inhibits fetal breathing movements (FBMs) and increases inflammation and PGE2 levels in brainstem respiratory centers, cerebrospinal fluid (CSF) and blood plasma.
Methods: Chronically instrumented late preterm fetal sheep at 0.85 of gestation were randomly assigned to receive repeated intravenous saline (n = 8) or lipopolysaccharide (LPS) infusions (experimental day 1 = 300 ng, day 2 = 600 ng, day 3 = 1200 ng, n = 8). Fetal breathing movements were recorded throughout the experimental period. Sheep were euthanized 4 days after starting infusions for assessment of brainstem respiratory center histology.
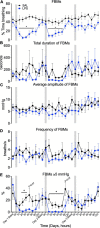
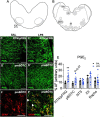
Results: LPS infusions increased circulating and cerebrospinal fluid PGE2 levels, decreased arterial oxygen saturation, increased the partial pressure of carbon dioxide and lactate concentration, and decreased pH (p < 0.05 for all) compared to controls. LPS infusions caused transient reductions in the % of time fetuses spent breathing and the proportion of vigorous fetal breathing movements (P < 0.05 vs. control). LPS-exposure increased PGE2 expression in the RTN/pFRG (P < 0.05 vs. control) but not the pBÖTC (P < 0.07 vs. control) of the brainstem. No significant changes in gene expression were observed for PGE2 enzymes or caspase 3. LPS-exposure reduced the numbers of GFAP-immunoreactive astrocytes in the RTN/pFRG, NTS and XII of the brainstem (P < 0.05 vs. control for all) and increased microglial activation in the RTN/pFRG, preBÖTC, NTS, and XII brainstem respiratory centers (P < 0.05 vs. control for all).
Conclusion: Chronic LPS-exposure in late preterm fetal sheep increased PGE2 levels within the brainstem, CSF and plasma, and was associated with inhibition of FBMs, astrocyte loss and microglial activation within the brainstem respiratory centers. Further studies are needed to determine whether the inflammation-induced increase in PGE2 levels plays a key role in depressing respiratory drive in the perinatal period.
Keywords: PGE2; brainstem; fetal breathing movements; inflammation; respiratory centers.
Copyright © 2022 Stojanovska, Atta, Kelly, Zahra, Matthews-Staindl, Nitsos, Moxham, Pham, Hooper, Herlenius, Galinsky and Polglase.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Ibuprofen Does Not Prevent Inhibition of Fetal Breathing Movements Caused by Intrauterine Inflammation in Fetal Sheep.Int J Mol Sci. 2025 Jun 11;26(12):5591. doi: 10.3390/ijms26125591. Int J Mol Sci. 2025. PMID: 40565054 Free PMC article.
-
Tumor necrosis factor inhibition attenuates white matter gliosis after systemic inflammation in preterm fetal sheep.J Neuroinflammation. 2020 Mar 23;17(1):92. doi: 10.1186/s12974-020-01769-6. J Neuroinflammation. 2020. PMID: 32293473 Free PMC article.
-
Interleukin-1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near-term fetal sheep.J Neuroinflammation. 2021 Aug 31;18(1):189. doi: 10.1186/s12974-021-02238-4. J Neuroinflammation. 2021. PMID: 34465372 Free PMC article.
-
The central control of fetal breathing and skeletal muscle movements.J Physiol. 1984 Jan;346:1-18. doi: 10.1113/jphysiol.1984.sp015003. J Physiol. 1984. PMID: 6422029 Free PMC article. Review.
-
The Consequences of Preterm Birth and Chorioamnionitis on Brainstem Respiratory Centers: Implications for Neurochemical Development and Altered Functions by Inflammation and Prostaglandins.Front Cell Neurosci. 2018 Feb 1;12:26. doi: 10.3389/fncel.2018.00026. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29449803 Free PMC article. Review.
Cited by
-
The effect of histological and subclinical chorioamnionitis and funisitis on breathing effort in premature infants at birth: a retrospective cohort study.Eur J Pediatr. 2024 Dec;183(12):5497-5507. doi: 10.1007/s00431-024-05815-w. Epub 2024 Oct 25. Eur J Pediatr. 2024. PMID: 39453483 Free PMC article.
-
The influence of chorioamnionitis on respiratory drive and spontaneous breathing of premature infants at birth: a narrative review.Eur J Pediatr. 2024 Jun;183(6):2539-2547. doi: 10.1007/s00431-024-05508-4. Epub 2024 Apr 1. Eur J Pediatr. 2024. PMID: 38558311 Free PMC article. Review.
-
Intra-amniotic infection with Ureaplasma parvum causes serovar-dependent white matter damage in preterm fetal sheep.Brain Commun. 2025 May 24;7(3):fcaf182. doi: 10.1093/braincomms/fcaf182. eCollection 2025. Brain Commun. 2025. PMID: 40462859 Free PMC article.
-
Investigating pulmonary inflammation and injury after progressive systemic inflammation in preterm fetal sheep.Front Physiol. 2025 May 15;16:1542613. doi: 10.3389/fphys.2025.1542613. eCollection 2025. Front Physiol. 2025. PMID: 40443447 Free PMC article.
-
Mechanical ventilation induces brainstem inflammation in preterm fetal sheep.Front Pediatr. 2023 Oct 23;11:1225294. doi: 10.3389/fped.2023.1225294. eCollection 2023. Front Pediatr. 2023. PMID: 37936886 Free PMC article.
References
-
- Chow S. S. W., Le Marsney R., Creighton P., Kander V., Haslam R., Lui K. (2017). Report of the Australian and New Zealand Neonatal Network. Sydney, NSW: ANZNN.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

