Simulation Modeling of Reduced Glycosylation Effects on Potassium Channels of Mouse Cardiomyocytes
- PMID: 35309072
- PMCID: PMC8931503
- DOI: 10.3389/fphys.2022.816651
Simulation Modeling of Reduced Glycosylation Effects on Potassium Channels of Mouse Cardiomyocytes
Abstract
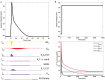
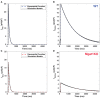
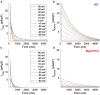
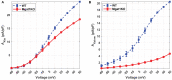
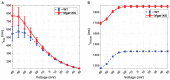
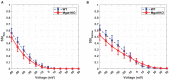
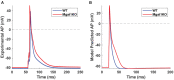
Dilated cardiomyopathy (DCM) is the third most common cause of heart failure and the primary reason for heart transplantation; upward of 70% of DCM cases are considered idiopathic. Our in-vitro experiments showed that reduced hybrid/complex N-glycosylation in mouse cardiomyocytes is linked with DCM. Further, we observed direct effects of reduced N-glycosylation on Kv gating. However, it is difficult to rigorously determine the effects of glycosylation on Kv activity, because there are multiple Kv isoforms in cardiomyocytes contributing to the cardiac excitation. Due to complex functions of Kv isoforms, only the sum of K+ currents (IKsum) can be recorded experimentally and decomposed later using exponential fitting to estimate component currents, such as IKto, IKslow, and IKss. However, such estimation cannot adequately describe glycosylation effects and Kv mechanisms. Here, we propose a framework of simulation modeling of Kv kinetics in mouse ventricular myocytes and model calibration using the in-vitro data under normal and reduced glycosylation conditions through ablation of the Mgat1 gene (i.e., Mgat1KO). Calibrated models facilitate the prediction of Kv characteristics at different voltages that are not directly observed in the in-vitro experiments. A model calibration procedure is developed based on the genetic algorithm. Experimental results show that, in the Mgat1KO group, both IKto and IKslow densities are shown to be significantly reduced and the rate of IKslow inactivation is much slower. The proposed approach has strong potential to couple simulation models with experimental data for gaining a better understanding of glycosylation effects on Kv kinetics.
Keywords: N-glycosylation; dilated cardiomyopathy; genetic algorithm; potassium channel; simulation modeling.
Copyright © 2022 Kim, Yang, Ednie and Bennett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
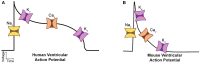

References
LinkOut - more resources
Full Text Sources

