The Interaction of Central Nervous System and Acute Kidney Injury: Pathophysiology and Clinical Perspectives
- PMID: 35309079
- PMCID: PMC8931545
- DOI: 10.3389/fphys.2022.826686
The Interaction of Central Nervous System and Acute Kidney Injury: Pathophysiology and Clinical Perspectives
Abstract
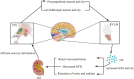
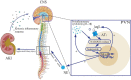
Acute kidney injury (AKI) is a common disorder in critically ill hospitalized patients. Its main pathological feature is the activation of the sympathetic nervous system and the renin-angiotensin system (RAS). This disease shows a high fatality rate. The reason is that only renal replacement therapy and supportive care can reduce the impact of the disease, but those measures cannot significantly improve the mortality. This review focused on a generalization of the interaction between acute kidney injury and the central nervous system (CNS). It was found that the CNS further contributes to kidney injury by regulating sympathetic outflow and oxidative stress in response to activation of the RAS and increased pro-inflammatory factors. Experimental studies suggested that inhibiting sympathetic activity and RAS activation in the CNS and blocking oxidative stress could effectively reduce the damage caused by AKI. Therefore, it is of significant interest to specify the mechanism on how the CNS affects AKI, as we could use such mechanism as a target for clinical interventions to further reduce the mortality and improve the complications of AKI. Systematic Review Registration: [www.ClinicalTrials.gov], identifier [registration number].
Keywords: acute kidney injury; central nervous system; neuromodulation; paraventricular hypothalamic; sympathetic nervous system.
Copyright © 2022 Wang, Liu, Liu and Lv.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aparicio-Trejo O. E., Avila-Rojas S. H., Tapia E., Rojas-Morales P., Leon-Contreras J. C., Martinez-Klimova E., et al. (2020). Chronic impairment of mitochondrial bioenergetics and beta-oxidation promotes experimental AKI-to-CKD transition induced by folic acid. Free Radic. Biol. Med. 154 18–32. 10.1016/j.freeradbiomed.2020.04.016 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

