AMEERA-5: a randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2- advanced breast cancer
- PMID: 35309087
- PMCID: PMC8928355
- DOI: 10.1177/17588359221083956
AMEERA-5: a randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2- advanced breast cancer
Abstract
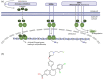
Background: For estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC), the current standard first-line treatment includes an aromatase inhibitor in combination with a cyclin-dependent kinase 4/6 inhibitor. When resistance occurs, often related to the occurrence of ESR1 mutations, selective estrogen receptor modulators or degraders (SERDs) may be used, alone or in combination regimens. Amcenestrant (SAR439859), an optimized oral SERD, has shown clinical antitumor activity in combination with palbociclib in patients with ER+/HER2- ABC and, as monotherapy, in patients with and without ESR1 mutations. Here, we describe the study design of AMEERA-5, an ongoing, prospective, phase 3, randomized, double-blind, multinational study comparing the efficacy and safety of amcenestrant plus palbociclib versus letrozole plus palbociclib in patients with advanced (locoregional recurrent or metastatic) ER+/HER2- breast cancer.
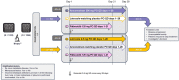
Methods: Patients are pre-/postmenopausal women and men with no prior systemic therapy for ABC. The planned enrollment is 1066 patients. Patients are randomized 1:1 to either amcenestrant 200 mg plus palbociclib 125 mg or letrozole 2.5 mg plus palbociclib 125 mg. Amcenestrant, letrozole, and their matching placebos are taken once daily continuously; palbociclib is taken once daily for 21 days, followed by 7 days off-treatment for a 28-day cycle. Treatment continues until disease progression, unacceptable toxicity, or decision to stop treatment. Pre-/perimenopausal women and men receive goserelin subcutaneously. Randomization is stratified by de novo metastatic disease, menopausal status, and visceral metastases. The primary endpoint is progression-free survival. The key secondary endpoint is overall survival; others are safety, pharmacokinetics, and quality of life.
Conclusions: AMEERA-5 is evaluating the efficacy and safety of amcenestrant in combination with palbociclib as first-line therapy in pre-/postmenopausal women and men with ER+/HER2- ABC.
Clinicaltrials identifier: NCT04478266.
Keywords: ER-positive/HER2-negative; amcenestrant; endocrine therapy; metastatic breast cancer; selective estrogen receptor degrader.
© The Author(s), 2022.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB reports consulting or advisory roles at Biotheranostics, Daiichi Sankyo/AstraZeneca, Foundation Medicine, Genentech, Immunomedics, Merck, Novartis, Pfizer, Philips, Puma Biotechnology, Radius Health, Sanofi, and Spectrum Pharmaceuticals; consulting or advisory roles (to his institution) with Genentech/Roche, Immunomedics, Innocrin Pharma, Novartis, Pfizer, and Radius Health; and research funding to his institution from AstraZeneca/Daiichi Sankyo, Genentech, Immunomedics, Merck, Novartis, Pfizer, Radius Health, and Sanofi. JC reports stock/ownership interest at MedSIR; honoraria from Celgene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung; consulting or advisory role at AstraZeneca, Athenex, Bioasis, Biothera, Boehringer Ingelheim, Celgene, Cellestia Biotech, Clovis Oncology, Daiichi Sankyo, ERYTECH Pharma, GlaxoSmithKline, Kyowa Kyrin, Leuko, Lilly, Merck Sharp & Dohme, Merus, Polyphor, Roche, Seattle Genetics, and SERVIER; and research funding to his institution from ARIAD, AstraZeneca, Baxalta, Bayer, Eisai, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur, Puma Biotechnology, Queen Mary University of London, and Roche; and travel/accommodations/expenses from Daiichi Sankyo, Eisai, Novartis, Pfizer, and Roche. SAH reports stock/ownership interests at Ideal Implant and ROM Tech; research funding to her institution from Ambryx, Amgen, Arvinas, Bayer, Biomarin, Cascadian Therapeutics, Daiichi Sankyo, Dignitana, Genentech/Roche, Gilead Sciences, GlaxoSmithKline, Immunomedics, Lilly, Macrogenics, Merrimack, Novartis, OBI Pharma, Pfizer, Phoenix Molecular Designs, Pieris Pharmaceuticals, Puma Biotechnology, Radius Health, Sanofi, Seattle Genetics, and Zymeworks; and travel/accommodations/expenses from Lilly; and other relationships with Pfizer and Roche. SD reports consulting or advisory roles (to her institution) with AstraZeneca and Pierre Fabre; research funding to her institution from AstraZeneca, Exact Sciences, Lilly, Novartis, Pfizer, Puma Biotechnology, Roche/Genentech, and Sanofi; and travel/accommodations/expenses from AstraZeneca, Pfizer, and Roche. HI reports honoraria from AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kirin, Lilly Japan, Pfizer, and Taiho Pharmaceutical; consulting or advisory roles with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly Japan, Novartis, and Pfizer; and research funding to his institution from AstraZeneca, Bayer, Boehringer Ingelheim, Chugai Pharma, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly Japan, MSD, Nihonkayaku, Novartis, Pfizer, and Sanofi. ZMS reports no disclosures. DK, PC, QL, and SCC are employees of Sanofi and may hold shares and/or stock options in the company. VP is a former employee of Sanofi and a current employee of Bayer. JO discloses honoraria from AbbVie, Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Genentech, Genomic Health, GRAIL, HERON, Immunomedics, Ipsen, Jounce Therapeutics, Lilly, Merck, Myriad Pharmaceuticals, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Samsung, Sanofi, Seattle Genetics, and Syndax; consulting or advisory roles with AbbVie, Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Genentech, Genomic Health, GRAIL, HERON, Immunomedics, Ipsen, Jounce Therapeutics, Lilly, Merck, Myriad Pharmaceuticals, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Samsung, Sanofi, Seattle Genetics, and Syndax; speakers’ bureau fees from AstraZeneca, Lilly, Novartis, and Pfizer; research funding to her institution from Seattle Genetics; and travel/accommodations/expenses from AbbVie, Agendia, Amgen, AstraZeneca, Celgene, Eisai, Genomic Health, GRAIL, Ipsen, Jounce Therapeutics, Lilly, Myriad Pharmaceuticals, Novartis, Pfizer, Puma Biotechnology, Roche, Sanofi, and Seattle Genetics.
Figures
Similar articles
-
Randomized Phase III Study of Amcenestrant Plus Palbociclib Versus Letrozole Plus Palbociclib in Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Primary Results From AMEERA-5.J Clin Oncol. 2024 Aug 1;42(22):2680-2690. doi: 10.1200/JCO.23.02036. Epub 2024 Jun 18. J Clin Oncol. 2024. PMID: 38889373 Clinical Trial.
-
AMEERA-3: Randomized Phase II Study of Amcenestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Monotherapy in Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer.J Clin Oncol. 2023 Aug 20;41(24):4014-4024. doi: 10.1200/JCO.22.02746. Epub 2023 Jun 22. J Clin Oncol. 2023. PMID: 37348019 Free PMC article. Clinical Trial.
-
The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study.Lancet Oncol. 2015 Jan;16(1):25-35. doi: 10.1016/S1470-2045(14)71159-3. Epub 2014 Dec 16. Lancet Oncol. 2015. PMID: 25524798 Clinical Trial.
-
Palbociclib: A Novel Cyclin-Dependent Kinase Inhibitor for Hormone Receptor-Positive Advanced Breast Cancer.Ann Pharmacother. 2015 Nov;49(11):1252-60. doi: 10.1177/1060028015602273. Epub 2015 Aug 31. Ann Pharmacother. 2015. PMID: 26324355 Free PMC article. Review.
-
Impact of palbociclib combinations on treatment of advanced estrogen receptor-positive/human epidermal growth factor 2-negative breast cancer.Onco Targets Ther. 2016 Oct 11;9:6119-6125. doi: 10.2147/OTT.S77033. eCollection 2016. Onco Targets Ther. 2016. PMID: 27785059 Free PMC article. Review.
Cited by
-
An emerging generation of endocrine therapies in breast cancer: a clinical perspective.NPJ Breast Cancer. 2023 Apr 5;9(1):20. doi: 10.1038/s41523-023-00523-4. NPJ Breast Cancer. 2023. PMID: 37019913 Free PMC article. Review.
-
Decoding estrogen receptor and GPER biology: structural insights and therapeutic advances in ERα-positive breast cancer.Front Oncol. 2025 Jun 26;15:1513225. doi: 10.3389/fonc.2025.1513225. eCollection 2025. Front Oncol. 2025. PMID: 40641933 Free PMC article. Review.
-
CDK4/6 Inhibitors Overcome Endocrine ESR1 Mutation-Related Resistance in Metastatic Breast Cancer Patients.Cancers (Basel). 2023 Feb 18;15(4):1306. doi: 10.3390/cancers15041306. Cancers (Basel). 2023. PMID: 36831647 Free PMC article.
-
The intersection of the HER2-low subtype with endocrine resistance: the role of interconnected signaling pathways.Front Oncol. 2024 Nov 22;14:1461190. doi: 10.3389/fonc.2024.1461190. eCollection 2024. Front Oncol. 2024. PMID: 39650068 Free PMC article.
-
Oral SERD, a Novel Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer.Cancers (Basel). 2024 Jan 31;16(3):619. doi: 10.3390/cancers16030619. Cancers (Basel). 2024. PMID: 38339371 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- American Society of Clinical Oncology (ASCO). Breast cancer: statistics, https://www.cancer.net/cancer-types/breast-cancer/statistics (2021, accessed 8 October 2021).
-
- Stravodimou A, Voutsadakis IA. The future of ER+/HER2- metastatic breast cancer therapy: beyond PI3K inhibitors. Anticancer Res 2020; 40: 4829–4841. - PubMed
-
- Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther 2018; 186: 1–24. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

