The role of macrophages in thermal injury
- PMID: 35309103
- PMCID: PMC8918762
The role of macrophages in thermal injury
Abstract
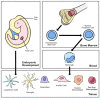
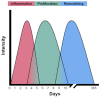
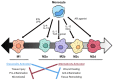
Macrophages, first discovered for their phagocytic ability, are a complicated and heterogeneous cell type. The unique properties of macrophages allow them to perform a vast array of functions, including phagocytosis, cytokine production, antigen presentation, and wound healing. Some macrophage populations are derived from monocytes and are induced into specific phenotypes by the local tissue microenvironment, while other macrophages form during early embryonic development. The exposure of the host to local pathogens and/or traumatic injury alters the tissue microenvironment and, in turn, influences changes in macrophage phenotype and function. Perhaps the most significant change in the local tissue microenvironment and subsequent macrophage phenotype occurs after thermal injury, which causes localized tissue damage and a massive systemic inflammatory response. However, few studies have explored the influence of burn injury on the host macrophages and macrophage function in burn wounds. Furthermore, the literature is scant regarding the impact macrophage function has on outcomes in thermal injury. This review will focus on the current knowledge of macrophage function in burn wounds and the phenotypic changes in macrophages during thermal injury while identifying knowledge gaps.
Keywords: Macrophages; macrophage dysfunction; macrophage phenotype; thermal injury; wound healing.
IJBT Copyright © 2022.
Conflict of interest statement
None.
Figures
Similar articles
-
Macrophages in skin injury and repair.Immunobiology. 2011 Jul;216(7):753-62. doi: 10.1016/j.imbio.2011.01.001. Epub 2011 Jan 8. Immunobiology. 2011. PMID: 21281986 Review.
-
Anti-inflammatory and burn injury wound healing properties of the shell of Haliotis diversicolor.BMC Complement Altern Med. 2016 Nov 28;16(1):487. doi: 10.1186/s12906-016-1473-6. BMC Complement Altern Med. 2016. PMID: 27894302 Free PMC article.
-
The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum.Am J Pathol. 1975 Jan;78(1):71-100. Am J Pathol. 1975. PMID: 1109560 Free PMC article.
-
Plasma extracellular vesicles released after severe burn injury modulate macrophage phenotype and function.J Leukoc Biol. 2022 Jan;111(1):33-49. doi: 10.1002/JLB.3MIA0321-150RR. Epub 2021 Aug 3. J Leukoc Biol. 2022. PMID: 34342045 Free PMC article.
-
The role of cardiac resident macrophage in cardiac aging.Aging Cell. 2023 Dec;22(12):e14008. doi: 10.1111/acel.14008. Epub 2023 Oct 10. Aging Cell. 2023. PMID: 37817547 Free PMC article. Review.
Cited by
-
4-aminopyridine attenuates inflammation and apoptosis and increases angiogenesis to promote skin regeneration following a burn injury.Res Sq [Preprint]. 2024 Aug 1:rs.3.rs-4669610. doi: 10.21203/rs.3.rs-4669610/v1. Res Sq. 2024. Update in: Cell Death Discov. 2024 Oct 4;10(1):428. doi: 10.1038/s41420-024-02199-6. PMID: 39149501 Free PMC article. Updated. Preprint.
-
Anti-inflammatory and antibacterial potential of Ajwa date (Phoenix dactylifera L.) extract in burn infection.J Adv Pharm Technol Res. 2023 Jul-Sep;14(3):161-165. doi: 10.4103/japtr.japtr_138_23. Epub 2023 Jul 28. J Adv Pharm Technol Res. 2023. PMID: 37692010 Free PMC article. Review.
-
4-aminopyridine attenuates inflammation and apoptosis and increases angiogenesis to promote skin regeneration following a burn injury in mice.Cell Death Discov. 2024 Oct 4;10(1):428. doi: 10.1038/s41420-024-02199-6. Cell Death Discov. 2024. PMID: 39366954 Free PMC article.
-
Accelerated Burn Healing in a Mouse Experimental Model Using α-Gal Nanoparticles.Bioengineering (Basel). 2023 Oct 6;10(10):1165. doi: 10.3390/bioengineering10101165. Bioengineering (Basel). 2023. PMID: 37892895 Free PMC article. Review.
-
Fighting Bleb Fibrosis After Glaucoma Surgery: Updated Focus on Key Players and Novel Targets for Therapy.Int J Mol Sci. 2025 Mar 5;26(5):2327. doi: 10.3390/ijms26052327. Int J Mol Sci. 2025. PMID: 40076946 Free PMC article.
References
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
-
- Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338–344. - PubMed
-
- Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–453. - PubMed
-
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
