Mass Spectrometry-Based Differentiation of Oral Tongue Squamous Cell Carcinoma and Nontumor Regions With the SpiderMass Technology
- PMID: 35309279
- PMCID: PMC8929397
- DOI: 10.3389/froh.2022.827360
Mass Spectrometry-Based Differentiation of Oral Tongue Squamous Cell Carcinoma and Nontumor Regions With the SpiderMass Technology
Abstract
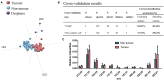
Oral cavity cancers are the 15th most common cancer with more than 350,000 new cases and ~178,000 deaths each year. Among them, squamous cell carcinoma (SCC) accounts for more than 90% of tumors located in the oral cavity and on oropharynx. For the oral cavity SCC, the surgical resection remains the primary course of treatment. Generally, surgical margins are defined intraoperatively using visual and tactile elements. However, in 15-30% of cases, positive margins are found after histopathological examination several days postsurgery. Technologies based on mass spectrometry (MS) were recently developed to help guide surgical resection. The SpiderMass technology is designed for in-vivo real-time analysis under minimally invasive conditions. This instrument achieves tissue microsampling and real-time molecular analysis with the combination of a laser microprobe and a mass spectrometer. It ultimately acts as a tool to support histopathological decision-making and diagnosis. This pilot study included 14 patients treated for tongue SCC (T1 to T4) with the surgical resection as the first line of treatment. Samples were first analyzed by a pathologist to macroscopically delineate the tumor, dysplasia, and peritumoral areas. The retrospective and prospective samples were sectioned into three consecutive sections and thaw-mounted on slides for H&E staining (7 μm), SpiderMass analysis (20 μm), and matrix-assisted laser desorption ionization (MALDI) MS imaging (12 μm). The SpiderMass microprobe collected lipidometabolic profiles of the dysplasia, tumor, and peritumoral regions annotated by the pathologist. The MS spectra were then subjected to the multivariate statistical analysis. The preliminary data demonstrate that the lipidometabolic molecular profiles collected with the SpiderMass are significantly different between the tumor and peritumoral regions enabling molecular classification to be established by linear discriminant analysis (LDA). MALDI images of the different samples were submitted to segmentation for cross instrument validation and revealed additional molecular discrimination within the tumor and nontumor regions. These very promising preliminary results show the applicability of the SpiderMass to SCC of the tongue and demonstrate its interest in the surgical treatment of head and neck cancers.
Keywords: decision support; head and neck cancer; lipidomics; mass spectrometry; precision surgery; real-time diagnosis; surgical margin; tongue squamous cell carcinoma.
Copyright © 2022 Ogrinc, Attencourt, Colin, Boudahi, Tebbakha, Salzet, Testelin, Dakpé and Fournier.
Conflict of interest statement
MS and IF are inventors of the patent (priority number WO2015IB57301 20150922) related to part of the described protocol. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

