HIV-gp140-Specific Antibodies Generated From Indian Long-Term Non-Progressors Mediate Potent ADCC Activity and Effectively Lyse Reactivated HIV Reservoir
- PMID: 35309295
- PMCID: PMC8924355
- DOI: 10.3389/fimmu.2022.844610
HIV-gp140-Specific Antibodies Generated From Indian Long-Term Non-Progressors Mediate Potent ADCC Activity and Effectively Lyse Reactivated HIV Reservoir
Abstract
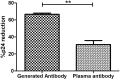
Strategies to reduce the human immunodeficiency virus (HIV) reservoir are urgently required. The antibody-dependent cellular cytotoxicity (ADCC)-mediating anti-HIV antibodies have shown an association with HIV control. We assessed if such antibodies can be generated in vitro and whether the generated antibodies can facilitate the reduction of reactivated HIV reservoir. We isolated HIV-1-gp140-specific memory B cells from HIV-1-infected long-term non-progressors (LTNPs) with or without plasma ADCC and cultured them to generate anti-HIV antibodies. The ability of the generated antibodies to mediate ADCC and facilitate NK cell-mediated lysis of reactivated HIV reservoir was assessed by the rapid fluorometric antibody-dependent cellular cytotoxicity assay and a flow-based novel latency reduction assay, respectively. All LTNPs showed the presence of gp140-specific memory B cells [median: 0.79% (0.54%-1.225%)], which were successfully differentiated into plasma cells [median 72.0% (68.7-82.2%)] in an in-vitro culture and secreted antibodies [median OD: 0.253 (0.205-0.274)]. The HIV-gp140-specific antibodies were generated from 11/13 LTNPs irrespective of their plasma ADCC status. The generated antibodies from LTNPs with plasma ADCC showed higher ADCC potency (median: 37.6%, IQR: 32.95%-51%) and higher reduction in reactivated HIV reservoir (median: 62.5%, IQR: 58.71%-64.92%) as compared with the antibodies generated from LTNPs without plasma ADCC (ADCC: median: 8.85%, IQR: 8%-9.7%; and % p24 reduction median: 13.84, IQR: 9.863%-17.81%). The potency of these antibodies to reduce latent reservoir was two-fold higher than the respective plasma ADCC. The study showed that the potent ADCC-mediating antibodies could be generated from memory B cells of the LTNPs with plasma ADCC activity. These antibodies also showed potent ability to facilitate NK cell-mediated lysis of reactivated HIV reservoirs. It also indicated that memory B cells from individuals with plasma ADCC activity should be preferentially used for such antibody generation. The important role of these antibodies in the reduction of latent reservoirs needs to be further evaluated as a useful strategy to obtain a functional cure for HIV infection.
Keywords: HIV-gp140 antibodies; HIV-specific memory B cells; antibody-dependent cellular cytotoxicity (ADCC); long-term non-progressors; reactivated latent reservoirs.
Copyright © 2022 Dhande, Bagul and Thakar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Anti-HIV-1 ADCC and HIV-1 Env Can Be Partners in Reducing Latent HIV Reservoir.Front Immunol. 2021 Apr 30;12:663919. doi: 10.3389/fimmu.2021.663919. eCollection 2021. Front Immunol. 2021. PMID: 33995393 Free PMC article.
-
Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: relevance to global HIV vaccine design.AIDS. 2014 Aug 24;28(13):1859-70. doi: 10.1097/QAD.0000000000000310. AIDS. 2014. PMID: 24937308
-
Potent In Vivo NK Cell-Mediated Elimination of HIV-1-Infected Cells Mobilized by a gp120-Bispecific and Hexavalent Broadly Neutralizing Fusion Protein.J Virol. 2017 Sep 27;91(20):e00937-17. doi: 10.1128/JVI.00937-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28794022 Free PMC article.
-
HIV-specific antibody-dependent cellular cytotoxicity: a novel vaccine modality.Expert Rev Clin Immunol. 2012 Nov;8(8):767-74. doi: 10.1586/eci.12.74. Expert Rev Clin Immunol. 2012. PMID: 23167688 Review.
-
Unlocking HIV-1 Env: implications for antibody attack.AIDS Res Ther. 2017 Sep 12;14(1):42. doi: 10.1186/s12981-017-0168-5. AIDS Res Ther. 2017. PMID: 28893275 Free PMC article. Review.
Cited by
-
Profiling HIV1-host protein-protein interaction networks in patient-derived exosome proteins: impact on pathophysiology and innate immune pathways.Virol J. 2025 Jul 28;22(1):259. doi: 10.1186/s12985-025-02717-7. Virol J. 2025. PMID: 40722180 Free PMC article.
-
Complement contributes to antibody-mediated protection against repeated SHIV challenge.Proc Natl Acad Sci U S A. 2023 May 16;120(20):e2221247120. doi: 10.1073/pnas.2221247120. Epub 2023 May 8. Proc Natl Acad Sci U S A. 2023. PMID: 37155897 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

