Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity
- PMID: 35309296
- PMCID: PMC8927970
- DOI: 10.3389/fimmu.2022.812774
Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity
Abstract
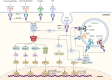
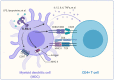
Innate immunity is the first defense system against invading pathogens. Toll-like receptors (TLRs) are well-defined pattern recognition receptors responsible for pathogen recognition and induction of innate immune responses. Since their discovery, TLRs have revolutionized the field of immunology by filling the gap between the initial recognition of pathogens by innate immune cells and the activation of the adaptive immune response. TLRs critically link innate immunity to adaptive immunity by regulating the activation of antigen-presenting cells and key cytokines. Furthermore, recent studies also have shown that TLR signaling can directly regulate the T cell activation, growth, differentiation, development, and function under diverse physiological conditions. This review provides an overview of TLR signaling pathways and their regulators and discusses how TLR signaling, directly and indirectly, regulates cell-mediated immunity. In addition, we also discuss how TLR signaling is critically important in the host's defense against infectious diseases, autoimmune diseases, and cancer.
Keywords: T cells; autoimmune diseases; cancer; cell-mediated immunity; infectious diseases; signaling pathway; toll-like receptors.
Copyright © 2022 Duan, Du, Xing, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

