Monitoring of Circulating CAR T Cells: Validation of a Flow Cytometric Assay, Cellular Kinetics, and Phenotype Analysis Following Tisagenlecleucel
- PMID: 35309367
- PMCID: PMC8926389
- DOI: 10.3389/fimmu.2022.830773
Monitoring of Circulating CAR T Cells: Validation of a Flow Cytometric Assay, Cellular Kinetics, and Phenotype Analysis Following Tisagenlecleucel
Abstract
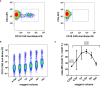
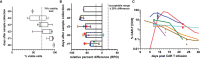
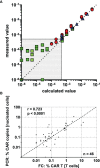
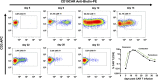
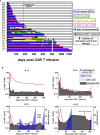
Chimeric antigen receptor (CAR) T cell therapy is a potent new treatment option for relapsed or refractory hematologic malignancies. As the monitoring of CAR T cell kinetics can provide insights into the activity of the therapy, appropriate CAR T cell detection methods are essential. Here, we report on the comprehensive validation of a flow cytometric assay for peripheral blood CD19 CAR T cell detection. Further, a retrospective analysis (n = 30) of CAR T cell and B cell levels over time has been performed, and CAR T cell phenotypes have been characterized. Serial dilution experiments demonstrated precise and linear quantification down to 0.05% of T cells or 22 CAR T cell events. The calculated detection limit at 13 events was confirmed with CAR T cell negative control samples. Inter-method comparison with real-time PCR showed appreciable correlation. Stability testing revealed diminished CAR T cell values already one day after sample collection. While we found long-term CAR T cell detectability and B cell aplasia in most patients (12/17), some patients (5/17) experienced B cell recovery. In three of these patients the coexistence of CAR T cells and regenerating B cells was observed. Repeat CAR T cell infusions led to detectable but limited re-expansions. Comparison of CAR T cell subsets with their counterparts among all T cells showed a significantly higher percentage of effector memory T cells and a significantly lower percentage of naïve T cells and T EMRA cells among CAR T cells. In conclusion, flow cytometric CAR T cell detection is a reliable method to monitor CAR T cells if measurements start without delay and sufficient T cell counts are given.
Keywords: acute lymphoblastic leukemia; chimeric antigen receptor (CAR); flow cytometry; immune monitoring; immunotherapy.
Copyright © 2022 Peinelt, Bremm, Kreyenberg, Cappel, Banisharif-Dehkordi, Erben, Rettinger, Jarisch, Meisel, Schlegel, Beck, Bug, Klusmann, Klingebiel, Huenecke and Bader.
Conflict of interest statement
PB declares research grants from Neovii, Riemser, Medac (to Institution); advisory board for Novartis, Cellgene, Amgen, Medac, Servier (personal and to Institution); Speakers Bureau of Miltenyi, Jazz, Riemser, Novartis, Amgen (to Institution), and patent and royalties from Medac. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, et al. Long-Term Outcome in Children With Relapsed Acute Lymphoblastic Leukemia After Time-Point and Site-of-Relapse Stratification and Intensified Short-Course Multidrug Chemotherapy: Results of Trial ALL-REZ BFM 90. J Clin Oncol (2010) 28:2339–47. doi: 10.1200/JCO.2009.25.1983 - DOI - PubMed
-
- Sadelain M, Brentjens R, Rivière I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov (2013) 3:388–98. doi: 10.1158/2159-8290.CD-12-0548 - DOI - PMC - PubMed
-
- Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric Receptors Providing Both Primary and Costimulatory Signaling in T Cells From a Single Gene Product. J Immunol (1998) 161:2791–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

