Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells
- PMID: 35309732
- PMCID: PMC8928819
- DOI: 10.1080/2162402X.2022.2052418
Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells
Abstract
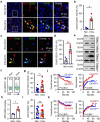
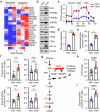
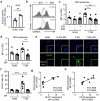
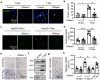
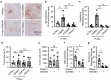
Neutrophils constitute a major component in human hepatocellular carcinoma (HCC) and can facilitate disease progression via poorly understood mechanisms. Here, we show that neutrophil extracellular traps (NETs) formation was increased in human HCC tumor tissues than in paired non-tumor liver tissues. Mechanism study revealed that tumor-induced metabolic switch toward glycolysis and pentose phosphate pathway in tumor infiltrating neutrophils promoted NETs formation in a reactive oxygen species dependent-manner. NETs subsequently induced the migration of cancer cells and down-regulation of tight junction molecules on adjacent endothelial cells, thus facilitating tumor intravasation and metastasis. Accordingly, NETs depletion could inhibit tumor metastasis in mice in vivo, and the infiltration levels of NETs-releasing neutrophils were negatively associated with patient survival and positively correlated with tumor metastasis potential of HCC patients. Our results unveiled a pro-metastatic role of NETs in the milieu of human HCC, and pointed to the importance of metabolic reprogramming in shaping their characteristics, thus providing an applicable efficient target for anti-cancer therapies.
Keywords: Neutrophils; hepatocellular carcinoma; metabolic switch; neutrophil-extracellular traps; tumor metastasis.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
