Neurons Release Injured Mitochondria as "Help-Me" Signaling After Ischemic Stroke
- PMID: 35309888
- PMCID: PMC8926840
- DOI: 10.3389/fnagi.2022.785761
Neurons Release Injured Mitochondria as "Help-Me" Signaling After Ischemic Stroke
Abstract
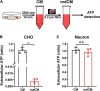
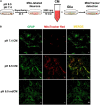
Mitochondrial dysfunction has been regarded as one of the major contributors of ischemic neuronal death after stroke. Recently, intercellular mitochondrial transfer between different cell types has been widely studied and suggested as a potential therapeutic approach. However, whether mitochondria are involved in the neuron-glia cross-talk following ischemic stroke and the underlying mechanisms have not been explored yet. In this study, we demonstrated that under physiological condition, neurons release few mitochondria into the extracellular space, and the mitochondrial release increased when subjected to the challenges of acidosis, hydrogen peroxide (H2O2), N-methyl-D-aspartate (NMDA), or glutamate. Acidosis reduced the mitochondrial basal respiration and lowered the membrane potential in primary-cultured mouse cortical neurons. These defective mitochondria were prone to be expelled to the extracellular space by the injured neurons, and were engulfed by adjacent astrocytes, leading to increased astrocytic expressions of mitochondrial Rho GTPase 1 (Miro 1) and mitochondrial transcription factor A (TFAM) at mRNA level. In mice subjected to transient focal cerebral ischemia, the number of defective mitochondria in the cerebrospinal fluid increased. Our results suggested that the neuron-derived mitochondria may serve as a "help-me" signaling and mediate the neuron-astrocyte cross-talk following ischemic stroke. Promoting the intercellular mitochondrial transfer by accelerating the neuronal releasing or astrocytic engulfing might be a potential and attractive therapeutic strategy for the treatment of ischemic stroke in the future.
Keywords: ischemic stroke; metabolic stress; mitochondrial biogenesis; mitochondrial release; neuron-glial crosstalk.
Copyright © 2022 Gao, Liu, Hou, Manaenko, Xiao, Wang, Xu and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

