Abnormalities in Cortical GABAergic Interneurons of the Primary Motor Cortex Caused by Lis1 (Pafah1b1) Mutation Produce a Non-drastic Functional Phenotype
- PMID: 35309904
- PMCID: PMC8924048
- DOI: 10.3389/fcell.2022.769853
Abnormalities in Cortical GABAergic Interneurons of the Primary Motor Cortex Caused by Lis1 (Pafah1b1) Mutation Produce a Non-drastic Functional Phenotype
Abstract
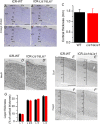
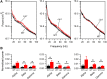
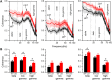
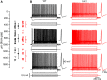
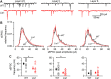
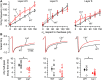
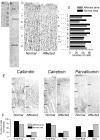
LIS1 (PAFAH1B1) plays a major role in the developing cerebral cortex, and haploinsufficient mutations cause human lissencephaly type 1. We have studied morphological and functional properties of the cerebral cortex of mutant mice harboring a deletion in the first exon of the mouse Lis1 (Pafah1b1) gene, which encodes for the LisH domain. The Lis1/sLis1 animals had an overall unaltered cortical structure but showed an abnormal distribution of cortical GABAergic interneurons (those expressing calbindin, calretinin, or parvalbumin), which mainly accumulated in the deep neocortical layers. Interestingly, the study of the oscillatory activity revealed an apparent inability of the cortical circuits to produce correct activity patterns. Moreover, the fast spiking (FS) inhibitory GABAergic interneurons exhibited several abnormalities regarding the size of the action potentials, the threshold for spike firing, the time course of the action potential after-hyperpolarization (AHP), the firing frequency, and the frequency and peak amplitude of spontaneous excitatory postsynaptic currents (sEPSC's). These morphological and functional alterations in the cortical inhibitory system characterize the Lis1/sLis1 mouse as a model of mild lissencephaly, showing a phenotype less drastic than the typical phenotype attributed to classical lissencephaly. Therefore, the results described in the present manuscript corroborate the idea that mutations in some regions of the Lis1 gene can produce phenotypes more similar to those typically described in schizophrenic and autistic patients and animal models.
Keywords: cortical inhibition; fast-spiking interneuron; mental disorders; motor cortex; oscillatory activity.
Copyright © 2022 Domínguez-Sala, Valdés-Sánchez, Canals, Reiner, Pombero, García-López, Estirado, Pastor, Geijo-Barrientos and Martínez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Properties of the epileptiform activity in the cingulate cortex of a mouse model of LIS1 dysfunction.Brain Struct Funct. 2022 Jun;227(5):1599-1614. doi: 10.1007/s00429-022-02458-1. Epub 2022 Feb 1. Brain Struct Funct. 2022. PMID: 35103859 Free PMC article.
-
Interneuron Heterotopia in the Lis1 Mutant Mouse Cortex Underlies a Structural and Functional Schizophrenia-Like Phenotype.Front Cell Dev Biol. 2021 Jul 13;9:693919. doi: 10.3389/fcell.2021.693919. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34327202 Free PMC article.
-
Developmental alterations of the septohippocampal cholinergic projection in a lissencephalic mouse model.Exp Neurol. 2015 Sep;271:215-27. doi: 10.1016/j.expneurol.2015.06.014. Epub 2015 Jun 14. Exp Neurol. 2015. PMID: 26079645
-
Clinical and molecular basis of classical lissencephaly: Mutations in the LIS1 gene (PAFAH1B1).Hum Mutat. 2002 Jan;19(1):4-15. doi: 10.1002/humu.10028. Hum Mutat. 2002. PMID: 11754098 Review.
-
Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex.J Neurocytol. 2002 Mar-Jun;31(3-5):277-87. doi: 10.1023/a:1024126110356. J Neurocytol. 2002. PMID: 12815247 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

