Biochemical Characterization of a Human Septin Octamer
- PMID: 35309913
- PMCID: PMC8928218
- DOI: 10.3389/fcell.2022.771388
Biochemical Characterization of a Human Septin Octamer
Abstract
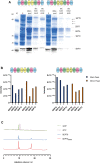
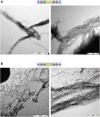
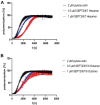
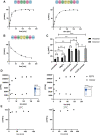
Septins are part of the cytoskeleton and polymerize into non-polar filaments of heteromeric hexamers or octamers. They belong to the class of P-loop GTPases but the roles of GTP binding and hydrolysis on filament formation and dynamics are not well understood. The basic human septin building block is the septin rod, a hetero-octamer composed of SEPT2, SEPT6, SEPT7, and SEPT9 with a stoichiometry of 2:2:2:2 (2-6-7-9-9-7-6-2). Septin rods polymerize by end-to-end and lateral joining into linear filaments and higher ordered structures such as rings, sheets, and gauzes. We purified a recombinant human septin octamer from E. coli for in vitro experimentation that is able to polymerize into filaments. We could show that the C-terminal region of the central SEPT9 subunit contributes to filament formation and that the human septin rod decreases the rate of in vitro actin polymerization. We provide further first kinetic data on the nucleotide uptake- and exchange properties of human hexameric and octameric septin rods. We could show that nucleotide uptake prior to hydrolysis is a dynamic process and that a bound nucleotide is exchangeable. However, the hydrolyzed γ-phosphate is not released from the native protein complex. We consequently propose that GTP hydrolysis in human septins does not follow the typical mechanism known from other small GTPases.
Keywords: GTPases; actin polymerization; nucleotide uptake; septin octamer; septins.
Copyright © 2022 Fischer, Frank, Rösler, Johnsson and Gronemeyer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bertin A., McMurray M. A., Grob P., Park S.-S., Garcia G., 3rd, Patanwala I., et al. (2008). Saccharomyces Cerevisiae Septins: Supramolecular Organization of Heterooligomers and the Mechanism of Filament Assembly. Proc. Natl. Acad. Sci. 105 (24), 8274–8279. 10.1073/pnas.0803330105 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

