Enriched Environment and Exercise Enhance Stem Cell Therapy for Stroke, Parkinson's Disease, and Huntington's Disease
- PMID: 35309929
- PMCID: PMC8927702
- DOI: 10.3389/fcell.2022.798826
Enriched Environment and Exercise Enhance Stem Cell Therapy for Stroke, Parkinson's Disease, and Huntington's Disease
Abstract
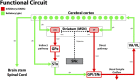
Stem cells, specifically embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), induced pluripotent stem cells (IPSCs), and neural progenitor stem cells (NSCs), are a possible treatment for stroke, Parkinson's disease (PD), and Huntington's disease (HD). Current preclinical data suggest stem cell transplantation is a potential treatment for these chronic conditions that lack effective long-term treatment options. Finding treatments with a wider therapeutic window and harnessing a disease-modifying approach will likely improve clinical outcomes. The overarching concept of stem cell therapy entails the use of immature cells, while key in recapitulating brain development and presents the challenge of young grafted cells forming neural circuitry with the mature host brain cells. To this end, exploring strategies designed to nurture graft-host integration will likely enhance the reconstruction of the elusive neural circuitry. Enriched environment (EE) and exercise facilitate stem cell graft-host reconstruction of neural circuitry. It may involve at least a two-pronged mechanism whereby EE and exercise create a conducive microenvironment in the host brain, allowing the newly transplanted cells to survive, proliferate, and differentiate into neural cells; vice versa, EE and exercise may also train the transplanted immature cells to learn the neurochemical, physiological, and anatomical signals in the brain towards better functional graft-host connectivity.
Keywords: Parkinson’s disease; combination therapy; enriched enviroment; exercise; huntingtons’s disease; rehabiliatation; stem cell; stroke.
Copyright © 2022 Berlet, Galang Cabantan, Gonzales-Portillo and Borlongan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Akhoundzadeh K., Vakili A., Sameni H. R., Vafaei A. A., Rashidy-Pour A., Safari M., et al. (2017). Effects of the Combined Treatment of Bone Marrow Stromal Cells with Mild Exercise and Thyroid Hormone on Brain Damage and Apoptosis in a Mouse Focal Cerebral Ischemia Model. Metab. Brain Dis. 32 (4), 1267–1277. 10.1007/s11011-017-0034-0 - DOI - PubMed
-
- Armstrong M. J., Miyasaki J. M. American Academy of Neurology (2012). Evidence-based Guideline: Pharmacologic Treatment of Chorea in Huntington Disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 79 (6), 597–603. 10.1212/WNL.0b013e318263c443 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

