Towards Bioinspired Meniscus-Regenerative Scaffolds: Engineering a Novel 3D Bioprinted Patient-Specific Construct Reinforced by Biomimetically Aligned Nanofibers
- PMID: 35309966
- PMCID: PMC8932947
- DOI: 10.2147/IJN.S353937
Towards Bioinspired Meniscus-Regenerative Scaffolds: Engineering a Novel 3D Bioprinted Patient-Specific Construct Reinforced by Biomimetically Aligned Nanofibers
Abstract
Introduction: Three of the main requirements that remain major challenges in tissue engineering of the knee meniscus are to engineer scaffolds with compatible anatomical shape, good mechanical properties, and microstructure able to mimic the architecture of the extracellular matrix (ECM). In this context, we presented a new biofabrication strategy to develop a three-dimensional (3D) meniscus-regenerative scaffold with custom-made macroscopic size and microarchitecture bioinspired by the organization of structural fibers of native tissue ECM.
Methods: The concept was based on the combination of bioprinted cell-laden hydrogel (type 1 collagen) reinforced by multilayers of biomimetically aligned electrospun nanofibrous mats (polycaprolactone/carbon nanotubes, PCL/CNT), using a patient-specific 3D digital meniscus model reconstructed from MRI data by free and open-source software.
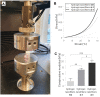
Results: The results showed that the incorporation of aligned nanofibers sheets between the hydrogel layers enhanced the scaffold's structural integrity and shape fidelity compared to the nanofiber-free collagen hydrogel. Furthermore, mechanical compression tests demonstrated that the presence of nanofiber layers significantly improved the mechanical properties of the bioprinted construct. Importantly, the introduction of PCL/CNT nanofibrous mats between the layers of the bioprinted collagen hydrogel did not negatively affect cell viability, in which mesenchymal stem cells remained viable even after 7 days of culture within the scaffold.
Conclusion: Overall, these findings evidence that this bioengineering approach offers a promising strategy for fabricating biomimetic meniscus scaffolds for tissue engineering.
Keywords: 3D printing; biomaterials; meniscus; nanotechnology; regenerative medicine; tissue engineering.
© 2022 Stocco et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
-
- Fox AJS, Wanivenhaus F, Burge AJ, Warren RF, Rodeo S. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat. 2015;28(2):269–287. - PubMed
-
- Markes AR, Hodax JD, Ma CB. Meniscus Form and Function. Clin Sports Med. 2020;39(1):1–12. - PubMed
-
- Palazzo C, Nguyen C, M-M L-C, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. - PubMed
-
- Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

