Therapeutic effects of asperosaponin VI in rabbit tendon disease
- PMID: 35310016
- PMCID: PMC8898761
- DOI: 10.1016/j.reth.2022.02.001
Therapeutic effects of asperosaponin VI in rabbit tendon disease
Abstract
Introduction: This study explored the effects and molecular mechanisms of asperosaponin VI in tendon disease.
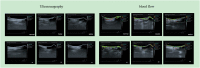
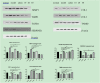
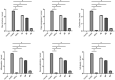
Methods: Forty-eight purebred adult male New Zealand white rabbits were randomly divided into the normal group (normal, n = 8); saline group (saline, n = 8) and prostaglandin E2 group (n = 32), which was further divided into four subgroups that were treated with asperosaponin VI doses of 0 mg/kg (model, n = 8), 10 mg/kg (10, n = 8), 20 mg/kg (20, n = 8) and 40 mg/kg (40, n = 8). The expression levels of matrix metallopeptidase 1 (MMP1), metallopeptidase inhibitor 1 (TIMP1), transforming growth factor beta 1 (TGFB1), serpin family E member 1 (SERPINE1), collagen Ⅰ (COL1), collagen Ⅲ (COL3) and tenomodulin (TNMD) in Achilles tendon tissue were determined through Western blot analysis. The histopathological changes in tendon tissue were observed by using Masson staining and haematoxylin-eosin staining.
Results: The expression levels of MMP1, TIMP1 and COL3 were higher and those of TGFB1, SERPINE1, COL1 and TNMD were lower in the 0 mg/kg group than in the normal group (P < 0.05). Compared with those in the 0 mg/kg group, the levels of MMP1 were lower in the 20 and 40 mg/kg groups. Compared with those in the 0 mg/kg group, the levels of TIMP1 were lower and the levels of TGFB1, COL1 and TNMD were higher in the 10, 20 and 40 mg/kg groups. In addition, compared with those in other groups, the levels of SERPINE1 in the 40 mg/kg group were significantly higher and the levels of COL3 in the 10 and 20 mg/kg groups were significantly lower (P < 0.05). Fibrous tissue arrangements and structures in the 40 mg/kg group were similar to those in the control group.
Conclusion: The effects of asperosaponin VI on injured tendons mainly involve eliminating inflammation, restoring balance to extracellular matrix collagen metabolism and inducing tendon cell proliferation. Asperosaponin VI is likely to be an ideal drug for the prevention and treatment of tendon disease.
Keywords: Achilles tendon disease; Asperosaponin Ⅵ; Collagen; Fibrosis; Healing; Proliferation.
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Hypoxia inducible factor-1α mediates the mechanism of the Hedgehog pathway in tendinopathy repair by Asperosaponin VI.Regen Ther. 2022 Oct 28;21:511-518. doi: 10.1016/j.reth.2022.10.008. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382134 Free PMC article. Review.
-
An asymmetric chitosan scaffold for tendon tissue engineering: In vitro and in vivo evaluation with rat tendon stem/progenitor cells.Acta Biomater. 2018 Jun;73:377-387. doi: 10.1016/j.actbio.2018.04.027. Epub 2018 Apr 17. Acta Biomater. 2018. PMID: 29678676
-
Tenomodulin regulates matrix remodeling of mouse tendon stem/progenitor cells in an ex vivo collagen I gel model.Biochem Biophys Res Commun. 2019 May 14;512(4):691-697. doi: 10.1016/j.bbrc.2019.03.063. Epub 2019 Mar 25. Biochem Biophys Res Commun. 2019. PMID: 30922565
-
Collagen and chondroitin sulfate functionalized bioinspired fibers for tendon tissue engineering application.Int J Biol Macromol. 2021 Feb 15;170:248-260. doi: 10.1016/j.ijbiomac.2020.12.152. Epub 2020 Dec 29. Int J Biol Macromol. 2021. PMID: 33359806
-
Matrix metabolism and healing in the flexor tendon. Experimental studies on rabbit tendon.Scand J Plast Reconstr Surg Hand Surg Suppl. 1991;23:1-51. Scand J Plast Reconstr Surg Hand Surg Suppl. 1991. PMID: 1947826 Review.
Cited by
-
Hypoxia inducible factor-1α mediates the mechanism of the Hedgehog pathway in tendinopathy repair by Asperosaponin VI.Regen Ther. 2022 Oct 28;21:511-518. doi: 10.1016/j.reth.2022.10.008. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382134 Free PMC article. Review.
-
Treatment options for Achilles tendinopathy: a scoping review of preclinical studies.PeerJ. 2025 Jan 10;13:e18143. doi: 10.7717/peerj.18143. eCollection 2025. PeerJ. 2025. PMID: 39807157 Free PMC article.
References
-
- Keefer H.M., Patterson C., Cuddeford T., et al. Low prevalence of patellar tendon abnormality and low incidence of patellar tendinopathy in female collegiate volleyball players. Res Sports Med. 2020;28(2):155–167. - PubMed
-
- Gaspar D., Spanoudes K. Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev. 2015;84:240–256. - PubMed
-
- Gleich J., Milz S., Ockert B. Principles of tendon healing at the shoulder and consequences for their treatment: importance of platelet-rich plasma and regenerative medicine. Unfallchirurg. 2021;124:89–95. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

