Recent Advances in the Elucidation of Frataxin Biochemical Function Open Novel Perspectives for the Treatment of Friedreich's Ataxia
- PMID: 35310092
- PMCID: PMC8924461
- DOI: 10.3389/fnins.2022.838335
Recent Advances in the Elucidation of Frataxin Biochemical Function Open Novel Perspectives for the Treatment of Friedreich's Ataxia
Abstract
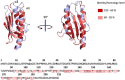
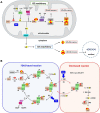
Friedreich's ataxia (FRDA) is the most prevalent autosomic recessive ataxia and is associated with a severe cardiac hypertrophy and less frequently diabetes. It is caused by mutations in the gene encoding frataxin (FXN), a small mitochondrial protein. The primary consequence is a defective expression of FXN, with basal protein levels decreased by 70-98%, which foremost affects the cerebellum, dorsal root ganglia, heart and liver. FXN is a mitochondrial protein involved in iron metabolism but its exact function has remained elusive and highly debated since its discovery. At the cellular level, FRDA is characterized by a general deficit in the biosynthesis of iron-sulfur (Fe-S) clusters and heme, iron accumulation and deposition in mitochondria, and sensitivity to oxidative stress. Based on these phenotypes and the proposed ability of FXN to bind iron, a role as an iron storage protein providing iron for Fe-S cluster and heme biosynthesis was initially proposed. However, this model was challenged by several other studies and it is now widely accepted that FXN functions primarily in Fe-S cluster biosynthesis, with iron accumulation, heme deficiency and oxidative stress sensitivity appearing later on as secondary defects. Nonetheless, the biochemical function of FXN in Fe-S cluster biosynthesis is still debated. Several roles have been proposed for FXN: iron chaperone, gate-keeper of detrimental Fe-S cluster biosynthesis, sulfide production stimulator and sulfur transfer accelerator. A picture is now emerging which points toward a unique function of FXN as an accelerator of a key step of sulfur transfer between two components of the Fe-S cluster biosynthetic complex. These findings should foster the development of new strategies for the treatment of FRDA. We will review here the latest discoveries on the biochemical function of frataxin and the implication for a potential therapeutic treatment of FRDA.
Keywords: Friedreich’s ataxia; frataxin; iron-sulfur cluster; persulfide; therapy.
Copyright © 2022 Monfort, Want, Gervason and D’Autréaux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

