An adhesive and resilient hydrogel for the sealing and treatment of gastric perforation
- PMID: 35310345
- PMCID: PMC8892218
- DOI: 10.1016/j.bioactmat.2021.11.038
An adhesive and resilient hydrogel for the sealing and treatment of gastric perforation
Abstract
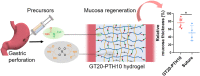
Adhesive hydrogels have been recently proposed as a potential option to seal and treat gastric perforation (GP) which causes high mortality despite advancements in surgical treatments. However, to be effective, the hydrogels must have sufficient tissue adhesiveness, tough mechanical property, tunable biodegradability and ideally are easy to apply and form. Herein, we report an adhesive and resilient hydrogel for the sealing and treatment of gastric perforation. The hydrogel consists of a bioactive, transglutaminase (TG)-crosslinked gelatin network and a dynamic, borate-crosslinked poly-N-[Tris(hydroxymethyl)methyl]acrylamide (PTH) network. The hydrogel can be formed in situ, facilitating easy delivery to the GP and allowing for precise sealing of the defects. In vivo experiments, using a perforated stomach mouse model, shows that the adhesive hydrogel plug effectively seals GP defects and promotes gastric mucosa regeneration. Overall, this hydrogel represents a promising biomaterial for GP treatment.
Keywords: Adhesive hydrogel plug; Gastric perforation; Regeneration; Thermo-responsive.
© 2022 The Authors.
Figures





Similar articles
-
Photocurable injectable Janus hydrogel with minimally invasive delivery for all-in-one treatment of gastric perforations and postoperative adhesions.Theranostics. 2023 Sep 25;13(15):5365-5385. doi: 10.7150/thno.87639. eCollection 2023. Theranostics. 2023. PMID: 37908723 Free PMC article.
-
Mussel-inspired poly(γ-gl utamic acid)/nanosilicate composite hydrogels with enhanced mechanical properties, tissue adhesive properties, and skin tissue regeneration.Acta Biomater. 2021 Mar 15;123:254-262. doi: 10.1016/j.actbio.2021.01.014. Epub 2021 Jan 16. Acta Biomater. 2021. PMID: 33465509
-
Magneto-Thermal Hydrogel Swarms for Targeted Lesion Sealing.Adv Healthc Mater. 2025 Jan;14(2):e2403076. doi: 10.1002/adhm.202403076. Epub 2024 Oct 24. Adv Healthc Mater. 2025. PMID: 39449232
-
Enzyme Catalyzed Hydrogel as Versatile Bioadhesive for Tissue Wound Hemostasis, Bonding, and Continuous Repair.Biomacromolecules. 2021 Apr 12;22(4):1346-1356. doi: 10.1021/acs.biomac.0c01329. Epub 2021 Mar 3. Biomacromolecules. 2021. PMID: 33657790
-
Robust hydrogel adhesives for emergency rescue and gastric perforation repair.Bioact Mater. 2022 May 14;19:703-716. doi: 10.1016/j.bioactmat.2022.05.010. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35633902 Free PMC article.
Cited by
-
Multi-targeted nanogel drug delivery system alleviates neuroinflammation and promotes spinal cord injury repair.Mater Today Bio. 2025 Jan 23;31:101518. doi: 10.1016/j.mtbio.2025.101518. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39935893 Free PMC article.
-
In situ 3D bioprinted GDMA/Prussian blue nanozyme hydrogel with wet adhesion promotes macrophage phenotype modulation and intestinal defect repair.Mater Today Bio. 2025 Mar 5;31:101636. doi: 10.1016/j.mtbio.2025.101636. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40161927 Free PMC article.
-
Multifunctional 3D platforms for rapid hemostasis and wound healing: Structural and functional prospects at biointerfaces.Int J Bioprint. 2022 Nov 29;9(1):648. doi: 10.18063/ijb.v9i1.648. eCollection 2023. Int J Bioprint. 2022. PMID: 36844240 Free PMC article.
-
Photocurable injectable Janus hydrogel with minimally invasive delivery for all-in-one treatment of gastric perforations and postoperative adhesions.Theranostics. 2023 Sep 25;13(15):5365-5385. doi: 10.7150/thno.87639. eCollection 2023. Theranostics. 2023. PMID: 37908723 Free PMC article.
-
Functional adhesive hydrogels for biological interfaces.Smart Med. 2023 Oct 7;2(4):e20230024. doi: 10.1002/SMMD.20230024. eCollection 2023 Nov. Smart Med. 2023. PMID: 39188302 Free PMC article. Review.
References
-
- Ramakrishnan K., Salinas R.C. Peptic ulcer disease. Am. Fam. Physician. 2007;76(7):1005–1012. - PubMed
-
- Najm W.I. Peptic ulcer disease. Prim. Care. 2011;38(3):383–394. - PubMed
-
- Tolentino L.E., Kallichanda N., Javier B., Yoshimori R., French S.W. A case report of gastric perforation and peritonitis associated with opportunistic infection by Sarcina ventriculi. Lab. Med. 2003;34(7):535–537.
LinkOut - more resources
Full Text Sources
Miscellaneous

