Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery
- PMID: 35310351
- PMCID: PMC8892166
- DOI: 10.1016/j.bioactmat.2021.11.018
Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery
Abstract
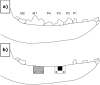
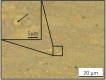
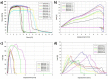
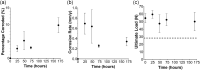
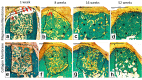
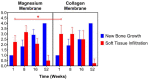
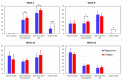
Barrier membranes are commonly used as part of the dental surgical technique guided bone regeneration (GBR) and are often made of resorbable collagen or non-resorbable materials such as PTFE. While collagen membranes do not provide sufficient mechanical protection of the covered bone defect, titanium reinforced membranes and non-resorbable membranes need to be removed in a second surgery. Thus, biodegradable GBR membranes made of pure magnesium might be an alternative. In this study a biodegradable pure magnesium (99.95%) membrane has been proven to have all of the necessary requirements for an optimal regenerative outcome from both a mechanical and biological perspective. After implantation, the magnesium membrane separates the regenerating bone from the overlying, faster proliferating soft tissue. During the initial healing period, the membrane maintained a barrier function and space provision, whilst retaining the positioning of the bone graft material within the defect space. As the magnesium metal corroded, it formed a salty corrosion layer and local gas cavities, both of which extended the functional lifespan of the membrane barrier capabilities. During the resorption of the magnesium metal and magnesium salts, it was observed that the membrane became surrounded and then replaced by new bone. After the membrane had completely resorbed, only healthy tissue remained. The in vivo performance study demonstrated that the magnesium membrane has a comparable healing response and tissue regeneration to that of a resorbable collagen membrane. Overall, the magnesium membrane demonstrated all of the ideal qualities for a barrier membrane used in GBR treatment.
Keywords: Biodegradable; Bone healing; GBR; GBR, Guided Bone Regeneration; Implant; Magnesium; Soft tissue healing.
© 2021 The Authors.
Conflict of interest statement
PR and ZP are employees of botiss biomaterials GmbH and FW is an employee of biotrics bioimplants AG.The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The following authors are employees of the company biotrics bioimplants AG (Frank Witte, Marco Bartosch) and botiss biomedical AG (Zeljka Peric Kacarevic, Patrick Rider, Drazen Tadic) which companies have financed the study. A CE mark has been successfully applied for the biodegradable magnesium barrier membrane using the published data in this manuscript.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

