Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives
- PMID: 35310361
- PMCID: PMC8892084
- DOI: 10.1016/j.bioactmat.2021.12.006
Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives
Abstract
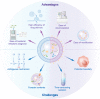
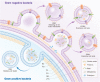
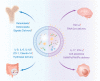
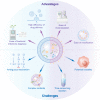
Nanosized extracellular vesicles derived from bacteria contain diverse cargo and transfer intercellular bioactive molecules to cells. Due to their favorable intercellular interactions, cell membrane-derived bacterial extracellular vesicles (BEVs) have great potential to become novel drug delivery platforms. In this review, we summarize the biogenesis mechanism and compositions of various BEVs. In addition, an overview of effective isolation and purification techniques of BEVs is provided. In particular, we focus on the application of BEVs as bioactive nanocarriers for drug delivery. Finally, we summarize the advances and challenges of BEVs after providing a comprehensive discussion in each section. We believe that a deeper understanding of BEVs will open new avenues for their exploitation in drug delivery applications.
Keywords: Bacterial extracellular vesicles; Bioactive nanocarriers; Drug delivery; Isolation and purification.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Nejman D., Livyatan I., Fuks G., Gavert N., Zwang Y., Geller L.T., Rotter-Maskowitz A., Weiser R., Mallel G., Gigi E., Meltser A., Douglas G.M., Kamer I., Gopalakrishnan V., Dadosh T., Levin-Zaidman S., Avnet S., Atlan T., Cooper Z.A., Arora R., Cogdill A.P., Khan M.A.W., Ologun G., Bussi Y., Weinberger A., Lotan-Pompan M., Golani O., Perry G., Rokah M., Bahar-Shany K., Rozeman E.A., Blank C.U., Ronai A., Shaoul R., Amit A., Dorfman T., Kremer R., Cohen Z.R., Harnof S., Siegal T., Yehuda-Shnaidman E., Gal-Yam E.N., Shapira H., Baldini N., Langille M.G.I., Ben-Nun A., Kaufman B., Nissan A., Golan T., Dadiani M., Levanon K., Bar J., Yust-Katz S., Barshack I., Peeper D.S., Raz D.J., Segal E., Wargo J.A., Sandbank J., Shental N., Straussman R. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. - PMC - PubMed
-
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19(1):55–71. - PubMed
-
- Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16(1):35–56. - PubMed
-
- Li S., Jiang W., Zheng C., Shao D., Liu Y., Huang S., Han J., Ding J., Tao Y., Li M. Oral delivery of bacteria: basic principles and biomedical applications. J. Contr. Release. 2020;327:801–833. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

