Local bone metabolism balance regulation via double-adhesive hydrogel for fixing orthopedic implants
- PMID: 35310387
- PMCID: PMC8897075
- DOI: 10.1016/j.bioactmat.2021.10.017
Local bone metabolism balance regulation via double-adhesive hydrogel for fixing orthopedic implants
Abstract
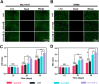
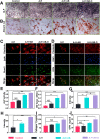
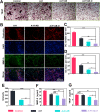
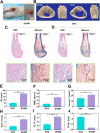
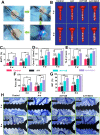
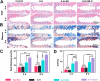
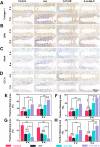
The effective osteointegration of orthopedic implants is a key factor for the success of orthopedic surgery. However, local metabolic imbalance around implants under osteoporosis condition could jeopardize the fixation effect. Inspired by the bone structure and the composition around implants under osteoporosis condition, alendronate (A) was grafted onto methacryloyl hyaluronic acid (H) by activating the carboxyl group of methacryloyl hyaluronic acid to be bonded to inorganic calcium phosphate on trabecular bone, which is then integrated with aminated bioactive glass (AB) modified by oxidized dextran (O) for further adhesion to organic collagen on the trabecular bone. The hybrid hydrogel could be solidified on cancellous bone in situ under UV irradiation and exhibits dual adhesion to organic collagen and inorganic apatite, promoting osteointegration of orthopedic implants, resulting in firm stabilization of the implants in cancellous bone areas. In vitro, the hydrogel was evidenced to promote osteogenic differentiation of embryonic mouse osteoblast precursor cells (MC3T3-E1) as well as inhibit the receptor activator of nuclear factor-κ B ligand (RANKL)-induced osteoclast differentiation of macrophages, leading to the upregulation of osteogenic-related gene and protein expression. In a rat osteoporosis model, the bone-implant contact (BIC) of the hybrid hydrogel group increased by 2.77, which is directly linked to improved mechanical stability of the orthopedic implants. Overall, this organic-inorganic, dual-adhesive hydrogel could be a promising candidate for enhancing the stability of orthopedic implants under osteoporotic conditions.
Keywords: Dual-functional; Hydrogels; Osseointegration; Osteoporosis; Peri-implant.
© 2021 The Authors.
Figures




Similar articles
-
Dual functional hydrogel of osteoclastic-inhibition and osteogenic-stimulation for osteoporotic bone defect regeneration.Mater Today Bio. 2025 Feb 5;31:101550. doi: 10.1016/j.mtbio.2025.101550. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40018058 Free PMC article.
-
The effects of implant topography on osseointegration under estrogen deficiency induced osteoporotic conditions: Histomorphometric, transcriptional and ultrastructural analysis.Acta Biomater. 2016 Sep 15;42:351-363. doi: 10.1016/j.actbio.2016.06.035. Epub 2016 Jun 29. Acta Biomater. 2016. PMID: 27375286
-
Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Local Osteoporotic Canine Mandible Model for the Evaluation of Peri-Implant Bone Regeneration.Tissue Eng Part C Methods. 2017 Nov;23(11):781-794. doi: 10.1089/ten.TEC.2017.0196. Epub 2017 Aug 24. Tissue Eng Part C Methods. 2017. PMID: 28741427
-
Adjuvant therapies of bone graft around non-cemented experimental orthopedic implants stereological methods and experiments in dogs.Acta Orthop Suppl. 2008 Aug;79(330):1-43. Acta Orthop Suppl. 2008. PMID: 19065776 Review.
-
Peri-implant osteogenesis in health and osteoporosis.Micron. 2005;36(7-8):630-44. doi: 10.1016/j.micron.2005.07.008. Epub 2005 Sep 6. Micron. 2005. PMID: 16182543 Review.
Cited by
-
Chirality-Induced Hydroxyapatite Manipulates Enantioselective Bone-Implant Interactions Toward Ameliorative Osteoporotic Osseointegration.Adv Sci (Weinh). 2025 Feb;12(8):e2411602. doi: 10.1002/advs.202411602. Epub 2024 Dec 31. Adv Sci (Weinh). 2025. PMID: 39738981 Free PMC article.
-
Harnessing hydrogen sulfide in injectable hydrogels that guide the immune response and osteoclastogenesis balance for osteoporosis treatment.Mater Today Bio. 2024 Nov 12;29:101338. doi: 10.1016/j.mtbio.2024.101338. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39649246 Free PMC article.
-
Long-term postoperative treatment of open fractures using in situ medicine delivery based on mesoporous MCM-41/hydrogel composites.RSC Adv. 2025 May 9;15(19):15266-15275. doi: 10.1039/d5ra00313j. eCollection 2025 May 6. RSC Adv. 2025. PMID: 40352383 Free PMC article.
-
Advances in Functionalized Nanoparticles for Osteoporosis Treatment.Int J Nanomedicine. 2025 Jun 20;20:7869-7891. doi: 10.2147/IJN.S519945. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40557247 Free PMC article. Review.
-
Functional adhesive hydrogels for biological interfaces.Smart Med. 2023 Oct 7;2(4):e20230024. doi: 10.1002/SMMD.20230024. eCollection 2023 Nov. Smart Med. 2023. PMID: 39188302 Free PMC article. Review.
References
-
- Liu Z., Tang M., Zhao J., Chai R., Kang J. Looking into the future: toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv. Mater. 2018;30 - PubMed
-
- Li J., Long Y., Yang F., Wei H., Zhang Z., Wang Y., Wang J., Li C., Carlos C., Dong Y., Wu Y., Cai W., Wang X. Multifunctional artificial artery from direct 3D printing with built-in ferroelectricity and tissue-matching modulus for real-time sensing and occlusion monitoring. Adv. Funct. Mater. 2020;30 - PMC - PubMed
-
- Vatankhah-Varnosfaderani M., Daniel W., Everhart M.H., Pandya A.A., Liang H., Matyjaszewski K., Dobrynin A.V., Sheiko S.S. Mimicking biological stress-strain behaviour with synthetic elastomers. Nature. 2017;549:497–501. - PubMed
LinkOut - more resources
Full Text Sources

