Exciton interactions in helical crystals of a hydrogen-bonded eumelanin monomer
- PMID: 35310511
- PMCID: PMC8864807
- DOI: 10.1039/d1sc06755a
Exciton interactions in helical crystals of a hydrogen-bonded eumelanin monomer
Abstract
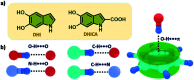
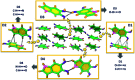
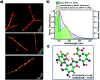
Eumelanin, a naturally occurring group of heterogeneous polymers/aggregates providing photoprotection to living organisms, consist of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) building blocks. Despite their prevalence in the animal world, the structure and therefore the mechanism behind the photoprotective broadband absorption and non-radiative decay of eumelanin remain largely unknown. As a small step towards solving the incessant mystery, DHI is crystallized in a non-protic solvent environment to obtain DHI crystals having a helical packing motif. The present approach reflects the solitary directional effect of hydrogen bonds between the DHI chromophores for generating the crystalline assembly and filters out any involvement of the surrounding solvent environment. The DHI single crystals having an atypical chiral packing motif (P212121 Sohncke space group) incorporate enantiomeric zig-zag helical stacks arranged in a herringbone fashion with respect to each other. Each of the zig-zag helical stacks originates from a bifurcated hydrogen bonding interaction between the hydroxyl substituents in adjacent DHI chromophores which act as the backbone structure for the helical assembly. Fragment-based excited state analysis performed on the DHI crystalline assembly demonstrates exciton delocalization along the DHI units that connect each enantiomeric helical stack while, within each stack, the excitons remain localized. Fascinatingly, over the time evolution for generation of single-crystals of the DHI-monomer, mesoscopic double-helical crystals are formed, possibly attributed to the presence of covalently connected DHI trimers in chloroform solution. The oligomeric DHI (in line with the chemical disorder model) along with the characteristic crystalline packing observed for DHI provides insights into the broadband absorption feature exhibited by the chromophore.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Electron diffraction and solid-state NMR reveal the structure and exciton coupling in a eumelanin precursor.Chem Sci. 2024 Sep 16;15(39):16015-24. doi: 10.1039/d4sc05453a. Online ahead of print. Chem Sci. 2024. PMID: 39345764 Free PMC article.
-
Hydration effects on the electronic properties of eumelanin building blocks.J Chem Phys. 2016 Aug 28;145(8):084501. doi: 10.1063/1.4961147. J Chem Phys. 2016. PMID: 27586929
-
Free Energy and Stacking of Eumelanin Nanoaggregates.J Phys Chem B. 2022 Mar 3;126(8):1805-1818. doi: 10.1021/acs.jpcb.1c07884. Epub 2022 Feb 17. J Phys Chem B. 2022. PMID: 35175060
-
Distinct Crystalline Aromatic Structural Motifs: Identification, Classification, and Implications.Acc Chem Res. 2019 Nov 19;52(11):3075-3086. doi: 10.1021/acs.accounts.9b00320. Epub 2019 Aug 26. Acc Chem Res. 2019. PMID: 31449389 Review.
-
"Fifty Shades" of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties.Int J Mol Sci. 2016 May 17;17(5):746. doi: 10.3390/ijms17050746. Int J Mol Sci. 2016. PMID: 27196900 Free PMC article. Review.
Cited by
-
Aggregation-assisted energy gap modulation controls delayed emission in hybrid charge-transfer emitters.Chem Sci. 2025 Jul 30. doi: 10.1039/d5sc02071a. Online ahead of print. Chem Sci. 2025. PMID: 40747320 Free PMC article.
-
Electron diffraction and solid-state NMR reveal the structure and exciton coupling in a eumelanin precursor.Chem Sci. 2024 Sep 16;15(39):16015-24. doi: 10.1039/d4sc05453a. Online ahead of print. Chem Sci. 2024. PMID: 39345764 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

