Exploratory imaging outcomes of a phase 1b/2a clinical trial of allopregnanolone as a regenerative therapeutic for Alzheimer's disease: Structural effects and functional connectivity outcomes
- PMID: 35310526
- PMCID: PMC8919249
- DOI: 10.1002/trc2.12258
Exploratory imaging outcomes of a phase 1b/2a clinical trial of allopregnanolone as a regenerative therapeutic for Alzheimer's disease: Structural effects and functional connectivity outcomes
Erratum in
-
Erratum.Alzheimers Dement (N Y). 2022 Jul 26;8(1):e12300. doi: 10.1002/trc2.12300. eCollection 2022. Alzheimers Dement (N Y). 2022. PMID: 35910666 Free PMC article.
Abstract
Introduction: Allopregnanolone (ALLO), an endogenous neurosteroid, promoted neurogenesis and oligogenesis and restored cognitive function in animal models of Alzheimer's disease (AD). Based on these discovery research findings, we conducted a randomized-controlled phase 1b/2a multiple ascending dose trial of ALLO in persons with early AD (NCT02221622) to assess safety, tolerability, and pharmacokinetics. Exploratory imaging outcomes to determine whether ALLO impacted hippocampal structure, white matter integrity, and functional connectivity are reported.
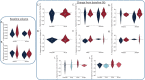
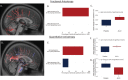
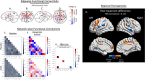
Methods: Twenty-four individuals participated in the trial (n = 6 placebo; n = 18 ALLO) and underwent brain magnetic resonance imaging (MRI) before and after 12 weeks of treatment. Hippocampal atrophy rate was determined from volumetric MRI, computed as rate of change, and qualitatively assessed between ALLO and placebo sex, apolipoprotein E (APOE) ε4 allele, and ALLO dose subgroups. White matter microstructural integrity was compared between placebo and ALLO using fractional and quantitative anisotropy (QA). Changes in local, inter-regional, and network-level functional connectivity were also compared between groups using resting-state functional MRI.
Results: Rate of decline in hippocampal volume was slowed, and in some cases reversed, in the ALLO group compared to placebo. Gain of hippocampal volume was evident in APOE ε4 carriers (range: 0.6% to 7.8% increased hippocampal volume). Multiple measures of white matter integrity indicated evidence of preserved or improved integrity. ALLO significantly increased fractional anisotropy (FA) in 690 of 690 and QA in 1416 of 1888 fiber tracts, located primarily in the corpus callosum, bilateral thalamic radiations, and bilateral corticospinal tracts. Consistent with structural changes, ALLO strengthened local, inter-regional, and network level functional connectivity in AD-vulnerable regions, including the precuneus and posterior cingulate, and network connections between the default mode network and limbic system.
Discussion: Indicators of regeneration from previous preclinical studies and these exploratory MRI-based outcomes from this phase 1b/2a clinical cohort support advancement to a phase 2 proof-of-concept efficacy clinical trial of ALLO as a regenerative therapeutic for mild AD (REGEN-BRAIN study; NCT04838301).
Keywords: Alzheimer's disease; allopregnanolone; functional connectivity; hippocampal volume; regenerative therapeutic; white matter integrity.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Adam C. Raikes reports no disclosures. Gerson D. Hernandez hold a leadership position with Neutherapeutics. Dawn C. Matthews is a paid consultant and CEO of ADM Diagnostics, Ana S. Lukic is VP of Research and Development for ADM Diagnostics, Meng Law reports no competing interests. Yonggang Shi reports no competing interests. Lon S. Schneider reports grants from Biogen, Roche/Genentech, Eli Lilly and Company, Novartis, and Biohaven; personal fees from Merck, Eli Lilly and Company, and Roche/Genentech for serving on the data and safety monitoring boards; personal fees from Takeda for serving as a consultant and on an adjudication committee; and consulting fees from AC Immune, Avraham Pharmaceuticals, Boehringer Ingelheim, Cognition Therapeutics, Cotexyme, Eisai, Neurim Pharmaceuticals, Neuronix, Tau RX, Toyama, Abbott, and vTv Therapeutics outside the submitted work. Robert D. Brinton holds a leadership position with Neutherapeutics; and patent US8969329B2 for allopregnanolone for the treatment of neurodegenerative diseases.
Figures
Similar articles
-
Safety, tolerability, and pharmacokinetics of allopregnanolone as a regenerative therapeutic for Alzheimer's disease: A single and multiple ascending dose phase 1b/2a clinical trial.Alzheimers Dement (N Y). 2020 Dec 16;6(1):e12107. doi: 10.1002/trc2.12107. eCollection 2020. Alzheimers Dement (N Y). 2020. PMID: 33344752 Free PMC article.
-
Apolipoprotein E ε4 Modulates Cognitive Profiles, Hippocampal Volume, and Resting-State Functional Connectivity in Alzheimer's Disease.J Alzheimers Dis. 2015;45(3):781-95. doi: 10.3233/JAD-142556. J Alzheimers Dis. 2015. PMID: 25624419
-
Aberrant Hippocampal Functional Connectivity Is Associated with Fornix White Matter Integrity in Alzheimer's Disease and Mild Cognitive Impairment.J Alzheimers Dis. 2020;75(4):1153-1168. doi: 10.3233/JAD-200066. J Alzheimers Dis. 2020. PMID: 32390630
-
Allopregnanolone as regenerative therapeutic for Alzheimer's disease: translational development and clinical promise.Prog Neurobiol. 2014 Feb;113:40-55. doi: 10.1016/j.pneurobio.2013.08.004. Epub 2013 Sep 14. Prog Neurobiol. 2014. PMID: 24044981 Free PMC article. Review.
-
Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative.Alzheimers Dement. 2019 Jan;15(1):106-152. doi: 10.1016/j.jalz.2018.08.005. Epub 2018 Oct 13. Alzheimers Dement. 2019. PMID: 30321505 Review.
Cited by
-
Allopregnanolone pleiotropic action in neurons and astrocytes: calcium signaling as a unifying mechanism.Front Endocrinol (Lausanne). 2023 Dec 22;14:1286931. doi: 10.3389/fendo.2023.1286931. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38189047 Free PMC article.
-
Emerging Pro-neurogenic Therapeutic Strategies for Neurodegenerative Diseases: A Review of Pre-clinical and Clinical Research.Mol Neurobiol. 2025 Jan;62(1):46-76. doi: 10.1007/s12035-024-04246-w. Epub 2024 May 31. Mol Neurobiol. 2025. PMID: 38816676 Free PMC article. Review.
-
Antiageing strategy for neurodegenerative diseases: from mechanisms to clinical advances.Signal Transduct Target Ther. 2025 Mar 10;10(1):76. doi: 10.1038/s41392-025-02145-7. Signal Transduct Target Ther. 2025. PMID: 40059211 Free PMC article. Review.
-
Neurosteroids Progesterone and Dehydroepiandrosterone: Molecular Mechanisms of Action in Neuroprotection and Neuroinflammation.Pharmaceuticals (Basel). 2025 Jun 23;18(7):945. doi: 10.3390/ph18070945. Pharmaceuticals (Basel). 2025. PMID: 40732235 Free PMC article. Review.
-
Allopregnanolone: Regenerative therapeutic to restore neurological health.Neurobiol Stress. 2022 Nov 5;21:100502. doi: 10.1016/j.ynstr.2022.100502. eCollection 2022 Nov. Neurobiol Stress. 2022. PMID: 36532370 Free PMC article.
References
-
- Association As . 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391‐460.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
