Application of predictive models in boosting power of Alzheimer's disease clinical trials: A post hoc analysis of phase 3 solanezumab trials
- PMID: 35310531
- PMCID: PMC8919041
- DOI: 10.1002/trc2.12223
Application of predictive models in boosting power of Alzheimer's disease clinical trials: A post hoc analysis of phase 3 solanezumab trials
Abstract
Background: The ideal participants for Alzheimer's disease (AD) clinical trials would show cognitive decline in the absence of treatment (i.e., placebo arm) and would also respond to the therapeutic intervention.
Objective: To investigate if predictive models can be an effective tool for identifying and excluding people unlikely to show cognitive decline as an enrichment strategy in AD trials.
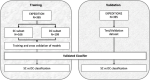
Method: We used data from the placebo arms of two phase 3, double-blind trials, EXPEDITION and EXPEDITION2. Patients had 18 months of follow-up. Based on the longitudinal data from the placebo arm, we classified participants into two groups: one showed cognitive decline (any negative slope) and the other showed no cognitive decline (slope is zero or positive) on the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-cog). We used baseline data for EXPEDITION to train regression-based classifiers and machine learning classifiers to estimate probability of cognitive decline. Models were applied to EXPEDITION2 data to assess predicted performance in an independent sample. Features used in predictive models included baseline demographics, apolipoprotein E ε4 genotype, neuropsychological scores, functional scores, and volumetric magnetic resonance imaging.
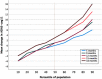
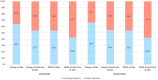
Result: In EXPEDITION, 46.3% of placebo-treated patients showed no cognitive decline and the proportion was similar in EXPEDITION2 (45.6%). Models had high sensitivity and modest specificity in both the training (EXPEDITION) and replication samples (EXPEDITION2) for detecting the stable group. Positive predictive value of models was higher than the base prevalence of cognitive decline, and negative predictive value of models were higher than the base rate of participants who had stable cognition.
Conclusion: Excluding persons with AD unlikely to decline from the active and placebo arms of clinical trials using predictive models may boost the power of AD trials through selective inclusion of participants expected to decline.
Keywords: Alzheimer's disease; anti‐amyloid monoclonal antibody; clinical trials; cognitive decline; machine learning; predictive analytics.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Ali Ezzati has served on the advisory board of Eisai. Christos Davatzikos receives research support from the following sources unrelated to this manuscript: NIH: R01NS042645, R01MH112070, R01NS042645, U24CA189523; and medical legal consulting work unrelated to this paper. David Wolk received grants from NIH, Merck, Biogen, and Eli Lilly. All payments have been to the University of Pennsylvania. He has received consulting fees from GE Healthcare and Neuronix and honoraria from MH Life Sciences. He also received Alzheimer's Association support to attend to AAIC in 2018 and 2019. He has served on advisory board of Functional Neuromodulation. He has received PET tracer from Eli Lilly for research (no payments were made). Charlie Hal receives research support from NIH and National Institute of Occupational Safety and Health for work unrelated to this manuscript. He receives payments from Washington University, St. Louis, for serving on External Advisory Committee for National Institute of Aging‐sponsored research, University of Iowa, for serving on Data Safety Monitoring Committee for National Institute of Aging‐sponsored research, from National Institutes of Health for serving on Study Section and Special Emphasis Panels for reviewing grant applications. He is also the unpaid director and secretary, Torah and Nature Foundation. His institution received loan of Thorasys tremoFlo C‐100 airwave oscillometer, Thorasys Thoracic Medical Systems Inc., www.thorasys.com, for a pilot study. The device was returned. Chris Habeck receives research support from NIA/NIH unrelated to this publication. He is an advisor for the Clinical trial “Motor Imagery Intervention” run by Helena Blumen at Albert Einstein College of Medicine, no payments are made. He received honorarium for Alzheimer's Research & Prevention Foundation Invited Presentation. Richard B. Lipton receives research support from the following sources unrelated to this manuscript: NIH: 2PO1 AG003949 (mPI), 5U10 NS077308 (PI), R21 AG056920 (Investigator), 1RF1 AG057531 (Site PI), RF1 AG054548 (Investigator), 1RO1 AG048642 (Investigator), R56 AG057548 (Investigator), U01062370 (Investigator), RO1 AG060933 (Investigator), RO1 AG062622 (Investigator), 1UG3FD006795 (mPI), 1U24NS113847 (Investigator), K23 NS09610 (Mentor), K23AG049466 (Mentor), K23 NS107643 (Mentor). He also receives support from the Migraine Research Foundation and the National Headache Foundation. He serves on the editorial board of Neurology, is a senior advisor to Headache, and associate editor for Cephalalgia. He has reviewed for the NIA and NINDS; holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from: Abbvie (Allergan), American Academy of Neurology, American Headache Society, Amgen, Avanir, Biohaven, Biovision, Boston Scientific, Reddy's (Promius), Electrocore, Eli Lilly, eNeura Therapeutics, Equinox, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, Vedanta. He receives royalties from Wolff's Headache seventh and eighth edition, Oxford Press University, 2009, Wiley and Informa. He receives consulting fees from Impel NeuroPharma and Novartis and has stock or options in Control M.
Figures
Similar articles
-
Machine Learning Predictive Models Can Improve Efficacy of Clinical Trials for Alzheimer's Disease.J Alzheimers Dis. 2020;74(1):55-63. doi: 10.3233/JAD-190822. J Alzheimers Dis. 2020. PMID: 31985462 Free PMC article.
-
Utilization of Observational Data as a Proxy Cohort for Comparison Purposes with Open-Label Study Results: An Example from Alzheimer's Disease.J Prev Alzheimers Dis. 2019;6(2):90-99. doi: 10.14283/jpad.2019.4. J Prev Alzheimers Dis. 2019. PMID: 30756115
-
Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients.Alzheimers Dement. 2016 Feb;12(2):110-120. doi: 10.1016/j.jalz.2015.06.1893. Epub 2015 Aug 1. Alzheimers Dement. 2016. PMID: 26238576 Clinical Trial.
-
Is the Alzheimer's Disease Assessment Scale-Cognitive Subscale Useful in Screening for Mild Cognitive Impairment and Alzheimer's Disease? A Systematic Review.Curr Alzheimer Res. 2022;19(3):202-211. doi: 10.2174/1567205019666220404104854. Curr Alzheimer Res. 2022. PMID: 35379127
-
The efficacy and safety of anti-Aβ agents for delaying cognitive decline in Alzheimer's disease: a meta-analysis.Front Aging Neurosci. 2023 Nov 6;15:1257973. doi: 10.3389/fnagi.2023.1257973. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38020763 Free PMC article.
Cited by
-
Association of Stages of Objective Memory Impairment With Incident Symptomatic Cognitive Impairment in Cognitively Normal Individuals.Neurology. 2023 May 30;100(22):e2279-e2289. doi: 10.1212/WNL.0000000000207276. Epub 2023 Apr 19. Neurology. 2023. PMID: 37076305 Free PMC article.
-
An explainable machine learning-based phenomapping strategy for adaptive predictive enrichment in randomized clinical trials.NPJ Digit Med. 2023 Nov 25;6(1):217. doi: 10.1038/s41746-023-00963-z. NPJ Digit Med. 2023. PMID: 38001154 Free PMC article.
References
-
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63‐75.e62. - PubMed
