ZEITLUPE Promotes ABA-Induced Stomatal Closure in Arabidopsis and Populus
- PMID: 35310670
- PMCID: PMC8924544
- DOI: 10.3389/fpls.2022.829121
ZEITLUPE Promotes ABA-Induced Stomatal Closure in Arabidopsis and Populus
Abstract
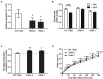
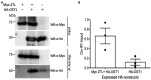
Plants balance water availability with gas exchange and photosynthesis by controlling stomatal aperture. This control is regulated in part by the circadian clock, but it remains unclear how signalling pathways of daily rhythms are integrated into stress responses. The serine/threonine protein kinase OPEN STOMATA 1 (OST1) contributes to the regulation of stomatal closure via activation of S-type anion channels. OST1 also mediates gene regulation in response to ABA/drought stress. We show that ZEITLUPE (ZTL), a blue light photoreceptor and clock component, also regulates ABA-induced stomatal closure in Arabidopsis thaliana, establishing a link between clock and ABA-signalling pathways. ZTL sustains expression of OST1 and ABA-signalling genes. Stomatal closure in response to ABA is reduced in ztl mutants, which maintain wider stomatal apertures and show higher rates of gas exchange and water loss than wild-type plants. Detached rosette leaf assays revealed a stronger water loss phenotype in ztl-3, ost1-3 double mutants, indicating that ZTL and OST1 contributed synergistically to the control of stomatal aperture. Experimental studies of Populus sp., revealed that ZTL regulated the circadian clock and stomata, indicating ZTL function was similar in these trees and Arabidopsis. PSEUDO-RESPONSE REGULATOR 5 (PRR5), a known target of ZTL, affects ABA-induced responses, including stomatal regulation. Like ZTL, PRR5 interacted physically with OST1 and contributed to the integration of ABA responses with circadian clock signalling. This suggests a novel mechanism whereby the PRR proteins-which are expressed from dawn to dusk-interact with OST1 to mediate ABA-dependent plant responses to reduce water loss in time of stress.
Keywords: OPEN STOMATA 1; PSEUDO-RESPONSE REGULATORs; ZEITLUPE; abiotic stress; abscisic acid; circadian clock; stomatal closure.
Copyright © 2022 Jurca, Sjölander, Ibáñez, Matrosova, Johansson, Kozarewa, Takata, Bakó, Webb, Israelsson-Nordström and Eriksson.
Conflict of interest statement
MEE is a member and CEO of the holding company Woodheads AB, a part-owner of SweTree Technologies (STT), which played no part in this work and she is also a board member of STT. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



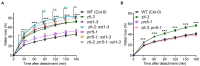

Similar articles
-
The key clock component ZEITLUPE (ZTL) negatively regulates ABA signaling by degradation of CHLH in Arabidopsis.Front Plant Sci. 2022 Sep 13;13:995907. doi: 10.3389/fpls.2022.995907. eCollection 2022. Front Plant Sci. 2022. PMID: 36176682 Free PMC article.
-
Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells.New Phytol. 2013 Dec;200(4):1049-63. doi: 10.1111/nph.12469. Epub 2013 Sep 3. New Phytol. 2013. PMID: 24033256
-
Plants lacking OST1 show conditional stomatal closure and wildtype-like growth sensitivity at high VPD.Physiol Plant. 2023 Sep-Oct;175(5):e14030. doi: 10.1111/ppl.14030. Physiol Plant. 2023. PMID: 37882302
-
Nitric oxide, stomatal closure, and abiotic stress.J Exp Bot. 2008;59(2):165-76. doi: 10.1093/jxb/erm293. J Exp Bot. 2008. PMID: 18332225 Review.
-
Beyond stress response: OST1 opening doors for plants to grow.Stress Biol. 2022 Oct 25;2(1):44. doi: 10.1007/s44154-022-00069-8. Stress Biol. 2022. PMID: 37676544 Free PMC article. Review.
Cited by
-
The circadian clock participates in seasonal growth in Norway spruce (Picea abies).Tree Physiol. 2024 Nov 5;44(11):tpae139. doi: 10.1093/treephys/tpae139. Tree Physiol. 2024. PMID: 39488796 Free PMC article.
-
Integration of light and ABA signaling pathways to combat drought stress in plants.Plant Cell Rep. 2023 May;42(5):829-841. doi: 10.1007/s00299-023-02999-7. Epub 2023 Mar 12. Plant Cell Rep. 2023. PMID: 36906730 Review.
-
The key clock component ZEITLUPE (ZTL) negatively regulates ABA signaling by degradation of CHLH in Arabidopsis.Front Plant Sci. 2022 Sep 13;13:995907. doi: 10.3389/fpls.2022.995907. eCollection 2022. Front Plant Sci. 2022. PMID: 36176682 Free PMC article.
-
The Regulatory Networks of the Circadian Clock Involved in Plant Adaptation and Crop Yield.Plants (Basel). 2023 May 6;12(9):1897. doi: 10.3390/plants12091897. Plants (Basel). 2023. PMID: 37176955 Free PMC article. Review.
-
Research progress on iron absorption, transport, and molecular regulation strategy in plants.Front Plant Sci. 2023 Jul 3;14:1190768. doi: 10.3389/fpls.2023.1190768. eCollection 2023. Front Plant Sci. 2023. PMID: 37465388 Free PMC article. Review.
References
-
- Bechtold N., Ellis J., Pelletier G. (1993). In-planta agrobacterium-mediated gene-transfer by infiltration of adult Arabidopsis thaliana plants. Compt. Rendus Acad. Sci. III Sci. Vie 316, 1194–1199.
LinkOut - more resources
Full Text Sources

