Endoscopic ultrasound-guided choledochoduodenostomy without fistula dilation using a stent with a 5.9-Fr delivery system: Comparison to a conventional procedure with fistula dilation
- PMID: 35310726
- PMCID: PMC8828169
- DOI: 10.1002/deo2.56
Endoscopic ultrasound-guided choledochoduodenostomy without fistula dilation using a stent with a 5.9-Fr delivery system: Comparison to a conventional procedure with fistula dilation
Abstract
Objectives: To evaluate the feasibility and safety of endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) without fistula dilation using a novel self-expandable metal stent (SEMS).
Methods: This retrospective study examined patients who underwent EUS-CDS for malignant distal biliary obstruction between October 2017 and May 2021 at the National Cancer Center, Japan. The primary outcome was a technical success without fistula dilation. Secondary outcomes were the overall technical success, clinical success, adverse events (AEs), procedure time, recurrent biliary obstruction (RBO), and time to RBO (TRBO).
Results: Forty-one patients were enrolled; 31 patients underwent EUS-CDS with fistula dilation using a conventional SEMS with 7.5-8.5-Fr delivery system (conventional SEMS group), and 10 patients underwent EUS-CDS without fistula dilation using the novel SEMS with a 5.9-Fr delivery system (novel SEMS group). In the novel SEMS group, the rate of technical success without fistula dilation was 90%. There were no differences in overall technical success (100% vs. 97%, p = 1.00), clinical success (80% vs. 90%, p = 0.58), and overall AEs (10% vs. 23%, p = 0.65) rates between the novel and conventional SEMS groups. In the novel SEMS group, no early AEs were observed and no bile leakage into the abdominal cavity was observed on the computed tomography scan after the procedure. The median procedure time was significantly shorter in the novel SEMS group (17 min vs. 24 min, p = 0.03). RBO and median TRBO did not differ between the 2 groups.
Conclusions: EUS-CDS without fistula dilation using the novel SEMS with a 5.9-Fr delivery system is technically feasible, straightforward, quick, and safe.
Keywords: EUS; EUS‐BD; EUS‐CDS; endoscopic ultrasound‐guided biliary drainage; endoscopic ultrasound‐guided choledochoduodenostomy.
© 2021 The Authors. DEN Open published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



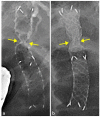
References
-
- Hara K, Yamao K, Niwa Y, et al. Prospective clinical study of EUS‐guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol 2011; 106: 1239–45. - PubMed
-
- Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound‐guided choledochoduodenostomy with direct metallic stent placement using a forward‐viewing echoendoscope. Endoscopy 2013; 45: 392–6. - PubMed
-
- Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest Endosc 2018; 88: 9–17. - PubMed
-
- Paik WH, Lee TH, Park DH, et al. EUS‐guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: A multicenter randomized clinical trial. Am J Gastroenterol 2018; 113: 987–97. - PubMed
-
- Park JK, Woo YS, Noh DH, et al. Efficacy of EUS‐guided and ERCP‐guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest Endosc 2018; 88: 277–82. - PubMed
LinkOut - more resources
Full Text Sources
