Long-term associations between amyloid positron emission tomography, sex, apolipoprotein E and incident dementia and mortality among individuals without dementia: hazard ratios and absolute risk
- PMID: 35310829
- PMCID: PMC8924651
- DOI: 10.1093/braincomms/fcac017
Long-term associations between amyloid positron emission tomography, sex, apolipoprotein E and incident dementia and mortality among individuals without dementia: hazard ratios and absolute risk
Abstract
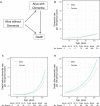
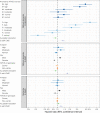
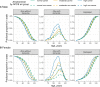
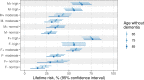
Dementia and mortality rates rise inexorably with age and consequently interact. However, because of the major logistical difficulties in accounting for both outcomes in a defined population, very little work has examined how risk factors and biomarkers for incident dementia are influenced by competing mortality. The objective of this study was to examine long-term associations between amyloid PET, APOE ɛ4, sex, education and cardiovascular/metabolic conditions, and hazard and absolute risk of dementia and mortality in individuals without dementia at enrolment. Participants were enrolled in the Mayo Clinic Study of Aging, a population-based study of cognitive ageing in Olmsted County, MN, USA. All were without dementia and were age 55-92 years at enrolment and were followed longitudinally. Predictor variables were amyloid PET, APOE ɛ4 status, sex, education, cardiovascular/metabolic conditions and age. The main outcomes were incident dementia and mortality. Multivariable, multi-state models were used to estimate mortality and incident dementia rates and absolute risk of dementia and mortality by predictor variable group. Of the 4984 participants in the study, 4336 (87%) were cognitively unimpaired and 648 (13%) had mild cognitive impairment at enrolment. The median age at enrolment was 75 years; 2463 (49%) were women. The median follow-up time was 9.4 years (7.5 years after PET). High versus normal amyloid (hazard ratio 2.11, 95% confidence interval 1.43-2.79), APOE ɛ4 (women: hazard ratio 2.24, 95% confidence interval 1.80-2.77; men: hazard ratio 1.37, 95% confidence interval 1.09-1.71), older age and two additional cardiovascular/metabolic conditions (hazard ratio 1.37, 95% confidence interval 1.22-1.53) were associated with the increased hazard of dementia (all P < 0.001). Among APOE ɛ4 carriers with elevated amyloid, remaining lifetime risk of dementia at age 65 years was greater in women [74% (95% confidence interval 65-84%) high and 58% (95% confidence interval 52-65%) moderate amyloid], than men [62% (95% confidence interval 52-73%) high and 44% (95% confidence interval 35-53%) moderate amyloid]. Overall, the hazard and absolute risk of dementia varied considerably by predictor group. The absolute risk of dementia associated with predictors characteristic of Alzheimer's disease was greater in women than men while at the same time the combination of APOE ɛ4 non-carrier with normal amyloid was more protective in women than men. This set of findings may be attributed in part to different biological effects and in part to lower mortality rates in women.
Keywords: APOE; amyloid PET; dementia; mortality; sex.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
