Novel Highlight in Malarial Drug Discovery: Aspartate Transcarbamoylase
- PMID: 35310840
- PMCID: PMC8931299
- DOI: 10.3389/fcimb.2022.841833
Novel Highlight in Malarial Drug Discovery: Aspartate Transcarbamoylase
Abstract
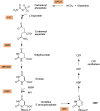
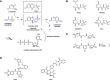
Malaria remains one of the most prominent and dangerous tropical diseases. While artemisinin and analogs have been used as first-line drugs for the past decades, due to the high mutational rate and rapid adaptation to the environment of the parasite, it remains urgent to develop new antimalarials. The pyrimidine biosynthesis pathway plays an important role in cell growth and proliferation. Unlike human host cells, the malarial parasite lacks a functional pyrimidine salvage pathway, meaning that RNA and DNA synthesis is highly dependent on the de novo synthesis pathway. Thus, direct or indirect blockage of the pyrimidine biosynthesis pathway can be lethal to the parasite. Aspartate transcarbamoylase (ATCase), catalyzes the second step of the pyrimidine biosynthesis pathway, the condensation of L-aspartate and carbamoyl phosphate to form N-carbamoyl aspartate and inorganic phosphate, and has been demonstrated to be a promising target both for anti-malaria and anti-cancer drug development. This is highlighted by the discovery that at least one of the targets of Torin2 - a potent, yet unselective, antimalarial - is the activity of the parasite transcarbamoylase. Additionally, the recent discovery of an allosteric pocket of the human homology raises the intriguing possibility of species selective ATCase inhibitors. We recently exploited the available crystal structures of the malarial aspartate transcarbamoylase to perform a fragment-based screening to identify hits. In this review, we summarize studies on the structure of Plasmodium falciparum ATCase by focusing on an allosteric pocket that supports the catalytic mechanisms.
Keywords: Plasmodium falciparum; X-ray structure; allosteric pocket; anti-malarials; aspartate transcarbamoylase; pyrimidine biosynthesis.
Copyright © 2022 Wang, Krüger, Du, Wrenger and Groves.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Crystal structure of truncated aspartate transcarbamoylase from Plasmodium falciparum.Acta Crystallogr F Struct Biol Commun. 2016 Jul;72(Pt 7):523-33. doi: 10.1107/S2053230X16008475. Epub 2016 Jun 22. Acta Crystallogr F Struct Biol Commun. 2016. PMID: 27380369 Free PMC article.
-
Identification of a non-competitive inhibitor of Plasmodium falciparum aspartate transcarbamoylase.Biochem Biophys Res Commun. 2018 Mar 11;497(3):835-842. doi: 10.1016/j.bbrc.2018.02.112. Epub 2018 Feb 21. Biochem Biophys Res Commun. 2018. PMID: 29476738
-
Exploring Aspartate Transcarbamoylase: A Promising Broad-Spectrum Target for Drug Development.Chembiochem. 2025 Apr 1;26(7):e202401009. doi: 10.1002/cbic.202401009. Epub 2025 Mar 27. Chembiochem. 2025. PMID: 39937588 Free PMC article. Review.
-
Molecular Target Validation of Aspartate Transcarbamoylase from Plasmodium falciparum by Torin 2.ACS Infect Dis. 2020 May 8;6(5):986-999. doi: 10.1021/acsinfecdis.9b00411. Epub 2020 Mar 12. ACS Infect Dis. 2020. PMID: 32129597
-
Structure and mechanisms of Escherichia coli aspartate transcarbamoylase.Acc Chem Res. 2012 Mar 20;45(3):444-53. doi: 10.1021/ar200166p. Epub 2011 Oct 19. Acc Chem Res. 2012. PMID: 22011033 Free PMC article. Review.
Cited by
-
Malaria therapeutics: are we close enough?Parasit Vectors. 2023 Apr 14;16(1):130. doi: 10.1186/s13071-023-05755-8. Parasit Vectors. 2023. PMID: 37060004 Free PMC article. Review.
-
Revisiting the Plasmodium falciparum druggable genome using predicted structures and data mining.Res Sq [Preprint]. 2024 Nov 26:rs.3.rs-5412515. doi: 10.21203/rs.3.rs-5412515/v1. Res Sq. 2024. Update in: NPJ Drug Discov. 2025;2(1):3. doi: 10.1038/s44386-025-00006-5. PMID: 39649165 Free PMC article. Updated. Preprint.
-
Revisiting the Plasmodium falciparum druggable genome using predicted structures and data mining.NPJ Drug Discov. 2025;2(1):3. doi: 10.1038/s44386-025-00006-5. Epub 2025 Mar 4. NPJ Drug Discov. 2025. PMID: 40066064 Free PMC article.
References
-
- Baillon J., Guichard M., Malaise E. P., Hervé G. (1983). Kinetic Parameters of Aspartate Transcarbamylase in Human Normal and Tumoral Cell Lines. Cancer Res. 43, 2277–2282. doi: 0008-5472/83/0043-0000$02.00 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

