Individual-level variations in malaria susceptibility and acquisition of clinical protection
- PMID: 35310901
- PMCID: PMC8914138
- DOI: 10.12688/wellcomeopenres.16524.3
Individual-level variations in malaria susceptibility and acquisition of clinical protection
Abstract
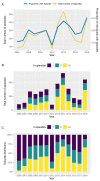
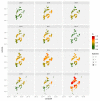
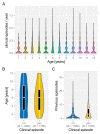
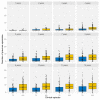
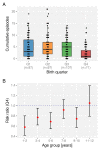
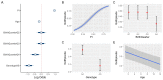
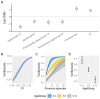
After decades of research, our understanding of when and why individuals infected with Plasmodium falciparum develop clinical malaria is still limited. Correlates of immune protection are often sought through prospective cohort studies, where measured host factors are correlated against the incidence of clinical disease over a set period of time. However, robustly inferring individual-level protection from these population-level findings has proved difficult due to small effect sizes and high levels of variance underlying such data. In order to better understand the nature of these inter-individual variations, we analysed the long-term malaria epidemiology of children ≤12 years old growing up under seasonal exposure to the parasite in the sub-location of Junju, Kenya. Despite the cohort's limited geographic expanse (ca. 3km x 10km), our data reveal a high degree of spatial and temporal variability in malaria prevalence and incidence rates, causing individuals to experience varying levels of exposure to the parasite at different times during their life. Analysing individual-level infection histories further reveal an unexpectedly high variability in the rate at which children experience clinical malaria episodes. Besides exposure to the parasite, measured as disease prevalence in the surrounding area, we find that the birth time of year has an independent effect on the individual's risk of experiencing a clinical episode. Furthermore, our analyses reveal that those children with a history of an above average number of episodes are more likely to experience further episodes during the upcoming transmission season. These findings are indicative of phenotypic differences in the rates by which children acquire clinical protection to malaria and offer important insights into the natural variability underlying malaria epidemiology.
Keywords: Plasmodium falciparum; clinical malaria; longitudinal cohort study; malaria susceptibility; naturally acquired immunity; spatial heterogeneity.
Copyright: © 2021 Valletta JJ et al.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

