Predictive energetic tuning of C-Nucleophiles for the electrochemical capture of carbon dioxide
- PMID: 35310940
- PMCID: PMC8927916
- DOI: 10.1016/j.isci.2022.103997
Predictive energetic tuning of C-Nucleophiles for the electrochemical capture of carbon dioxide
Abstract
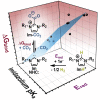
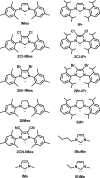
This work maps the thermodynamics of electrochemically generated C-nucleophiles for reactive capture of CO2. We identify a linear relationship between the pKa, the reduction potential of a protonated nucleophile (E red ), and the nucleophile's free energy of CO2 binding ( ). Through synergistic experiments and computations, this study establishes a three-parameter correlation described by the equation for a series of twelve imidazol(in)ium/N-heterocyclic carbene pairs with an R 2 of 0.92. The correlation allows us to predict the of C-nucleophiles to CO2 using reduction potentials or pKas of imidazol(in)ium cations. The carbenes in this study were found to exhibit a wide range CO2 binding strengths, from strongly CO2 binding to nonspontaneous. This observation suggests that the of imidazol(in)ium-based carbenes is tunable to a desired strength by appropriate structural changes. This work sets the stage for systematic energetic tuning of electrochemically enabled reactive separations.
Keywords: Applied chemistry; Chemistry; Computational chemistry; Electrochemistry; Theoretical chemistry.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Alherz A., Lim C.-H., Kuo Y.-C., Lehman P., Cha J., Hynes J.T., Musgrave C.B. Renewable hydride donors for the catalytic reduction of CO2: a thermodynamic and kinetic study. J. Phys. Chem. B. 2018;122:10179–10189. - PubMed
-
- Arduengo A.J., Davidson F., Dias H.V.R., Goerlich J.R., Khasnis D., Marshall W.J., Prakasha T.K. An air stable carbene and mixed carbene “dimers”. J. Am. Chem. Soc. 1997;119:12742–12749.
-
- Barone V., Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A. 1998;102:1995–2001.
-
- Camaioni D.M., Schwerdtfeger C.A. Comment on “accurate experimental values for the free energies of hydration of H+, OH-, and H3O+”. J. Phys. Chem. A. 2005;109:10795–10797. - PubMed
-
- Chai J.-D., Head-Gordon M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008;10:6615. - PubMed
LinkOut - more resources
Full Text Sources

