Airway Exposure to 1,3-Beta-d-Glucan Induces Airway Hyperresponsiveness in Guinea Pigs
- PMID: 35311019
- PMCID: PMC8922299
- DOI: 10.1021/acsptsci.1c00254
Airway Exposure to 1,3-Beta-d-Glucan Induces Airway Hyperresponsiveness in Guinea Pigs
Abstract
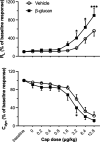
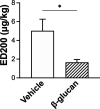
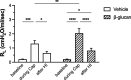
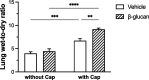
1,3-Beta-d-glucan (β-glucan) is a component of mold cell walls and is frequently found in fungi and house dust mites. The studies of β-glucan are inconsistent, although it has been implicated in airway adverse responses. This study was carried out to determine whether airway hyperresponsiveness was seen 24 h after airway exposure to β-glucan in guinea pigs. Two matching guinea pigs were exposed intratracheally to either β-glucan or its vehicle. Twenty-four hours after intratracheal instillation, there was no difference between these two groups in the baseline of the total pulmonary resistance (R L), dynamic lung compliance (C dyn), arterial blood pressure, and heart rate. In contrast, the responses of R L to capsaicin injection were significantly increased in β-glucan animals; capsaicin at the same dose of 3.2 μg/kg increased R L by 184% in vehicle animals and by 400% in β-glucan animals. The effective dose 200% to capsaicin injection was lower in the β-glucan animals. Furthermore, the increases in R L were partially reduced after transient lung hyperinflation to recruit the occluding airways; however, the R L induced by capsaicin injection after lung hyperinflation was significantly larger than the baseline in β-glucan animals; also, the lung wet-to-dry ratio in capsaicin-injected animals was augmented in the β-glucan group. Moreover, the airway hyperresponsiveness was accompanied by increases in neutrophils in the bronchoalveolar lavage fluid in the β-glucan animals. Furthermore, the levels of substance P and the calcitonin gene-related peptide in the bronchoalveolar lavage fluid collected after capsaicin injection were increased in β-glucan animals. We provide definitive evidence that β-glucan can induce airway hyperresponsiveness in guinea pigs, and the neuropeptide releases play an important role in this airway hyperresponsiveness.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
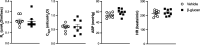




Similar articles
-
Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea pigs: role of tachykinins.J Appl Physiol (1985). 1999 Nov;87(5):1621-8. doi: 10.1152/jappl.1999.87.5.1621. J Appl Physiol (1985). 1999. PMID: 10562600
-
Involvement of Capsaicin-Sensitive Lung Vagal Neurons and TRPA1 Receptors in Airway Hypersensitivity Induced by 1,3-β-D-Glucan in Anesthetized Rats.Int J Mol Sci. 2020 Sep 18;21(18):6845. doi: 10.3390/ijms21186845. Int J Mol Sci. 2020. PMID: 32961891 Free PMC article.
-
Effects of capsaicin on the airway responses to inhaled endotoxin in the guinea pig.Am J Respir Crit Care Med. 1994 Jan;149(1):128-33. doi: 10.1164/ajrccm.149.1.8111569. Am J Respir Crit Care Med. 1994. PMID: 8111569
-
Airway hyperresponsiveness to cigarette smoke in ovalbumin-sensitized guinea pigs.Am J Respir Crit Care Med. 2000 Jan;161(1):73-80. doi: 10.1164/ajrccm.161.1.9809121. Am J Respir Crit Care Med. 2000. PMID: 10619800
-
Endogenous sensory neuropeptide release enhances nonspecific airway responsiveness in guinea pigs.Am Rev Respir Dis. 1992 Jul;146(1):148-53. doi: 10.1164/ajrccm/146.1.148. Am Rev Respir Dis. 1992. PMID: 1320818
References
LinkOut - more resources
Full Text Sources
