Cancer Neoantigens: Challenges and Future Directions for Prediction, Prioritization, and Validation
- PMID: 35311072
- PMCID: PMC8929516
- DOI: 10.3389/fonc.2022.836821
Cancer Neoantigens: Challenges and Future Directions for Prediction, Prioritization, and Validation
Abstract
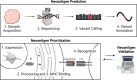
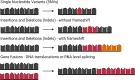
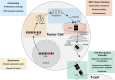
Prioritization of immunogenic neoantigens is key to enhancing cancer immunotherapy through the development of personalized vaccines, adoptive T cell therapy, and the prediction of response to immune checkpoint inhibition. Neoantigens are tumor-specific proteins that allow the immune system to recognize and destroy a tumor. Cancer immunotherapies, such as personalized cancer vaccines, adoptive T cell therapy, and immune checkpoint inhibition, rely on an understanding of the patient-specific neoantigen profile in order to guide personalized therapeutic strategies. Genomic approaches to predicting and prioritizing immunogenic neoantigens are rapidly expanding, raising new opportunities to advance these tools and enhance their clinical relevance. Predicting neoantigens requires acquisition of high-quality samples and sequencing data, followed by variant calling and variant annotation. Subsequently, prioritizing which of these neoantigens may elicit a tumor-specific immune response requires application and integration of tools to predict the expression, processing, binding, and recognition potentials of the neoantigen. Finally, improvement of the computational tools is held in constant tension with the availability of datasets with validated immunogenic neoantigens. The goal of this review article is to summarize the current knowledge and limitations in neoantigen prediction, prioritization, and validation and propose future directions that will improve personalized cancer treatment.
Keywords: MHC class I; MHC class II; neoantigen prediction; neoantigen prioritization; neoantigens (neoAgs).
Copyright © 2022 Borden, Buetow, Wilson and Hastings.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

