Treatment-Free Remission in Chronic Myeloid Leukemia Patients Treated With Low-Dose TKIs: A Feasible Option Also in the Real-Life. A Campus CML Study
- PMID: 35311109
- PMCID: PMC8927081
- DOI: 10.3389/fonc.2022.839915
Treatment-Free Remission in Chronic Myeloid Leukemia Patients Treated With Low-Dose TKIs: A Feasible Option Also in the Real-Life. A Campus CML Study
Abstract
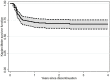
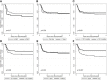
Treatment-free remission (TFR) has become a primary therapeutic goal in CML and is also considered feasible by international guidelines. TKIs dose reduction is often used in real-life practice to reduce adverse events, although its impact on TFR is still a matter of debate. This study aimed to explore the attitude of Italian hematologists towards prescribing TKIs at reduced doses and its impact on TFR. In September 2020, a questionnaire was sent to 54 hematology centers in Italy participating to the Campus CML network. For each patient, data on the main disease characteristics were collected. Most of the hematologists involved (64.4%) believed that low-dose TKIs should not influence TFR. Indeed, this approach was offered to 194 patients. At the time of TFR, all but 3 patients had already achieved a DMR, with a median duration of 61.0 months. After a median follow-up of 29.2 months, 138 (71.1%) patients were still in TFR. Interestingly, TFR outcome was not impaired by any of the variables examined, including sex, risk scores, BCR-ABL1 transcript types, previous interferon, type and number of TKIs used before treatment cessation, degree of DMR or median duration of TKIs therapy. On the contrary, TFR was significantly better after dose reduction due to AEs; furthermore, patients with a longer DMR duration showed a trend towards prolonged TFR. This survey indicates that low-dose TKI treatment is an important reality. While one third of Italian hematologists still had some uncertainties on TFR feasibility after using reduced doses of TKIs outside of clinical trials, TFR has often been considered a safe option even in patients treated with low-dose TKIs in the real-life setting. It should be noted that only 28.9% of our cases had a molecular recurrence, less than reported during standard dose treatment. Consequently, TFR is not impaired using low-dose TKIs.
Keywords: adverse event (AE); chronic myeloid leukemia; low dose; real life; treatment-free remission (TFR); tyrosine kinase inhibitors (TKI).
Copyright © 2022 Iurlo, Cattaneo, Artuso, Consonni, Abruzzese, Binotto, Bocchia, Bonifacio, Castagnetti, Galimberti, Gozzini, Iezza, Latagliata, Luciano, Maggi, Miggiano, Pregno, Rege-Cambrin, Russo, Scortechini, Tafuri, Tiribelli, Fava, Rosti, Foa, Breccia and Saglio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. . Adherence is the Critical Factor for Achieving Molecular Responses in Patients With Chronic Myeloid Leukemia Who Achieve Complete Cytogenetic Responses on Imatinib. J Clin Oncol (2010) 28:2381–8. doi: 10.1200/JCO.2009.26.3087 - DOI - PMC - PubMed

