Real-World First-Line Treatment Patterns and Outcomes in Hormone Receptor-Positive Advanced Breast Cancer Patients: A Multicenter, Retrospective Study in China
- PMID: 35311126
- PMCID: PMC8928103
- DOI: 10.3389/fonc.2022.829693
Real-World First-Line Treatment Patterns and Outcomes in Hormone Receptor-Positive Advanced Breast Cancer Patients: A Multicenter, Retrospective Study in China
Abstract
Background: Recent data on first-line treatment patterns administered to hormone receptor-positive (HR+) advanced breast cancer (ABC) patients in the real-world setting are limited. This study aimed to report the first-line treatment patterns and outcomes of HR+ ABC patients in China.
Methods: This was a multicenter, noninterventional study. Eligible patients were cytologically or histologically confirmed to have HR+ ABC with ≥2 complete medical records and received first-line therapies between January 2015 and June 2019. Treatment patterns and outcomes were extracted from structured or unstructured electronic medical records. Progression-free survival (PFS) was analyzed with the Kaplan-Meier method.
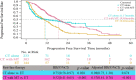
Results: In total, 1072 patients with HR+ ABC were enrolled at 6 treatment sites: 327 human epidermal growth factor receptor 2-positive (HER2+) patients, 696 HER2-negative (HER2-) patients and 49 HER2-unknown patients. Overall, 62.41% of patients received first-line chemotherapy (CT), 21.08% received targeted therapy (TT) and 15.49% received endocrine therapy (ET). For HR+/HER2+ patients, 65.14% received TT, 28.44% received CT, and 5.81% received ET. Compared with patients who received TT, patients who received CT alone, had a significantly worse median PFS (adjusted hazard ratio [HR] =2.59, 95% confidence interval [CI], 1.64-4.10, p<0.001). For HR+/HER2- patients, 77.01% received CT, 20.69% received ET and 1.15% received TT. Compared with patients who received ET, patients who received CT with maintenance therapy had a significantly prolonged median PFS (adjusted HR =0.57, 95% CI, 0.44-0.76, p<0.001). Among HR+/HER2- patients who received CT with maintenance treatment, those with maintenance ET had a longer median PFS than those with maintenance CT, but the difference was not significant (adjusted HR=0.92, 95% CI, 0.64-1.33, p=0.66).
Conclusions: This real-world study demonstrates that CT remains the mainstream first-line treatment option for HR+ patients in China. Among patients with HR+/HER2+ ABC, the majority received first-line TT and experienced a PFS benefit. A high percentage of HR+/HER2- patients received CT as first-line therapy in clinical practice. PFS benefit was significantly longer in patients who received CT with maintenance therapy. Moreover, there was no obvious difference in PFS between maintenance ET and CT. Maintenance ET may be a better choice considering its lower toxicity and better quality of life.
Keywords: advanced breast cancer; first-line treatment; hormone receptor-positive; outcomes; real-world study.
Copyright © 2022 Chen, Ouyang, Wang, Wang, Wang, Wu, Zhang, Huang, Zheng, Cao, Shao, Xie, Tian, Liang, Wang, Zhang, Ren and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Reviewers ZY and YS declared a past co-authorship with one of the authors QO to the handling editor.
Figures



Similar articles
-
Efficacy and safety of palbociclib plus endocrine therapy for patients with HR+/HER2- advanced breast cancer in real-world clinical practice.Ann Transl Med. 2022 Mar;10(6):362. doi: 10.21037/atm-22-1002. Ann Transl Med. 2022. PMID: 35434007 Free PMC article.
-
First-line treatment patterns and clinical outcomes in patients with HER2-positive and hormone receptor-positive metastatic breast cancer from registHER.Oncologist. 2013;18(5):501-10. doi: 10.1634/theoncologist.2012-0414. Epub 2013 May 7. Oncologist. 2013. PMID: 23652380 Free PMC article. Clinical Trial.
-
Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: Results from the EMERGE multicenter retrospective chart review study.BMC Cancer. 2019 Jan 18;19(1):88. doi: 10.1186/s12885-019-5301-5. BMC Cancer. 2019. PMID: 30658600 Free PMC article.
-
Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.Breast Cancer Res Treat. 2020 Feb;180(1):21-32. doi: 10.1007/s10549-020-05528-2. Epub 2020 Jan 22. Breast Cancer Res Treat. 2020. PMID: 31970560 Review.
-
Pharmacoeconomic evaluations of CDK4/6 inhibitors plus endocrine therapy for advanced hormone receptor-positive (HR+) and human epidermal growth factor receptor-2 negative (HER2-) breast cancer: a systematic review.Ann Transl Med. 2022 Feb;10(4):233. doi: 10.21037/atm-21-5110. Ann Transl Med. 2022. PMID: 35280368 Free PMC article. Review.
Cited by
-
Early Application of Palbociclib Plus Endocrine Therapy in HR+/HER2- Metastatic Breast Cancer: A Better Choice Based on Data From the Chinese Population.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221132926. doi: 10.1177/15330338221132926. Technol Cancer Res Treat. 2022. PMID: 36310472 Free PMC article.
-
RBP7 functions as a tumor suppressor in HR + breast cancer by inhibiting the AKT/SREBP1 pathway and reducing fatty acid.Cancer Cell Int. 2024 Mar 29;24(1):118. doi: 10.1186/s12935-024-03299-0. Cancer Cell Int. 2024. PMID: 38553715 Free PMC article.
-
Initial ribociclib plus endocrine therapy for HR+/HER2- advanced breast cancer in pre- and postmenopausal Chinese women: Primary results from a phase 2 randomized study.Cancer Med. 2024 Aug;13(15):e7408. doi: 10.1002/cam4.7408. Cancer Med. 2024. PMID: 39136200 Free PMC article. Clinical Trial.
-
An open-label, single-arm, multicenter, phase II trial of bireociclib as monotherapy for heavily pretreated HR-positive, HER2-negative advanced breast cancer patients: BRIGHT-1 trial.Cancer Commun (Lond). 2025 Jun;45(6):640-653. doi: 10.1002/cac2.70009. Epub 2025 Feb 27. Cancer Commun (Lond). 2025. PMID: 40013319 Free PMC article. Clinical Trial.
-
Clinical Prognosis and Nomograms for Hormone Receptor-Positive and Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Patients Treated with Palbociclib and Endocrine Therapy.Breast Cancer (Dove Med Press). 2025 Jul 24;17:669-681. doi: 10.2147/BCTT.S523199. eCollection 2025. Breast Cancer (Dove Med Press). 2025. PMID: 40735052 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

