Thymidine Kinase 1 Drives Skin Cutaneous Melanoma Malignant Progression and Metabolic Reprogramming
- PMID: 35311151
- PMCID: PMC8927676
- DOI: 10.3389/fonc.2022.802807
Thymidine Kinase 1 Drives Skin Cutaneous Melanoma Malignant Progression and Metabolic Reprogramming
Abstract
Background: Thymidine kinase 1 (TK1) is a cell cycle-dependent kinase that catalyzes the addition of a gamma-phosphate group to thymidine. The protumorigenic role of TK1 has been reported in various malignancies. However, the role of TK1 in skin cutaneous melanoma (SKCM) remains unclear. This study aimed to explore the molecular function of TK1 in SKCM progression.
Methods: Bioinformatics data were acquired from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO). Subcutaneous xenografts were established to observe the effect of TK1 knockdown on the proliferation of SKCM cells in vivo. RNA sequencing (RNA-seq; deposited in Sequence Read Archive, SRX10950283-SRX10950285 for A375 control cells and SRX10950286-SRX10950288 for TK1-silenced A375 cells) and immunoprecipitation-mass spectrometry (IP-MS) were used to analyze TK1-related genes and pathways. Seahorse XF Cell Mito tests and glycolysis stress assays were conducted for metabolic testing.
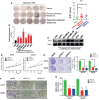
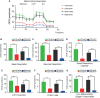
Results: TK1 was upregulated in malignant SKCM compared to that in normal tissues and cell lines. Elevated expression of TK1 was associated with poor prognosis. In vitro and in vivo assays demonstrated that TK1 promoted the proliferation and migration of SKCM cells. Moreover, TK1 was strongly associated with multiple intracellular metabolic pathways, facilitating cell mitochondrial respiration and glycolysis in SKCM malignant progression.
Conclusions: TK1 drives SKCM malignant progression and supports metabolic reprogramming, indicating that TK1 serves as a therapeutic target for SKCM.
Keywords: bioinformatics; cell metabolism; skin cutaneous melanoma; thymidine kinase 1; tumorigenesis.
Copyright © 2022 Zuo, Wang, Li, Pan and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

