Explosive Synchronization-Based Brain Modulation Reduces Hypersensitivity in the Brain Network: A Computational Model Study
- PMID: 35311218
- PMCID: PMC8927545
- DOI: 10.3389/fncom.2022.815099
Explosive Synchronization-Based Brain Modulation Reduces Hypersensitivity in the Brain Network: A Computational Model Study
Abstract
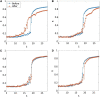
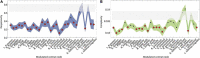
Fibromyalgia (FM) is a chronic pain condition that is characterized by hypersensitivity to multimodal sensory stimuli, widespread pain, and fatigue. We have previously proposed explosive synchronization (ES), a phenomenon wherein a small perturbation to a network can lead to an abrupt state transition, as a potential mechanism of the hypersensitive FM brain. Therefore, we hypothesized that converting a brain network from ES to general synchronization (GS) may reduce the hypersensitivity of FM brain. To find an effective brain network modulation to convert ES into GS, we constructed a large-scale brain network model near criticality (i.e., an optimally balanced state between order and disorders), which reflects brain dynamics in conscious wakefulness, and adjusted two parameters: local structural connectivity and signal randomness of target brain regions. The network sensitivity to global stimuli was compared between the brain networks before and after the modulation. We found that only increasing the local connectivity of hubs (nodes with intense connections) changes ES to GS, reducing the sensitivity, whereas other types of modulation such as decreasing local connectivity, increasing and decreasing signal randomness are not effective. This study would help to develop a network mechanism-based brain modulation method to reduce the hypersensitivity in FM.
Keywords: brain network control; brain network model; brain stimulation; chronic pain; explosive synchronization; hypersensitivity; state transition; stimulation.
Copyright © 2022 Kim, Harris, DaSilva and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bertotti G., Mayergoyz I. D. (2006). The Science of Hysteresis. Cambridge, MA: Academic Press.
-
- Boccaletti S., Almendral J. A., Guan S., Leyva I., Liu Z., Sendiña-Nadal I., et al. (2016). Explosive transitions in complex networks’ structure and dynamics: percolation and synchronization. Phys. Rep. 660 1–94. 10.1016/j.physrep.2016.10.004 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

