Cefiderocol- Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii
- PMID: 35311522
- PMCID: PMC9112922
- DOI: 10.1128/aac.02142-21
Cefiderocol- Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii
Abstract
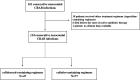
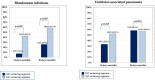
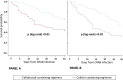
Cefiderocol may represent a therapeutic option for carbapenem-resistant Acinetobacter baumannii (CRAB) infections, but clinical data are limited. This is an observational retrospective study conducted in the University Hospital of Pisa including consecutive patients with CRAB infections (January 2020 to August 2021). Patients were divided in two study groups according to the antibiotic treatment received: cefiderocol- and colistin-containing regimens. The primary outcome was the 30-day mortality. A Cox regression analysis was performed to identify factors independently associated with 30-day mortality. A propensity score analysis using inverse probability of treatment weighting (IPTW) was also performed. A total of 124 patients were included: 47 (37.9%) received cefiderocol, while 77 (62.1%) colistin-containing regimens. Overall, 79 (63.7%) patients had a bloodstream infection (BSI), 35 (28.5%) a ventilator-associated pneumonia (VAP) and 10 (8.1%) other infections. Thirty-day mortality was higher in patients receiving colistin- compared to those who received cefiderocol-containing regimens (55.8% versus 34%, P = 0.018). This difference was confirmed in patients with BSI, but not in those with VAP. On multivariable analysis, septic shock, SOFA score, and age were independently associated with 30-day mortality, while cefiderocol therapy was protective in an IPTW analysis (Hazard ratio 0.44, 95% confidence interval 0.22-0.66, P < 0.001). Nephrotoxicity was more common in the colistin group. Microbiological failure occurred in 17.4% of patients receiving cefiderocol versus 6.8% of those receiving colistin (P = 0.079). Among 8 cases in the cefiderocol group who experienced microbiological failure, 4 (50%) developed resistance to cefiderocol. Cefiderocol represents a promising therapeutic option in patients with severe CRAB infections. Randomized clinical trial in this specific patient population should confirm our findings.
Keywords: Acinetobacter baumannii; antibiotic resistance; bloodstream infections; cefiderocol; colistin; multidrug resistance; pneumonia; sepsis; ventilator-associated pneumonia.
Conflict of interest statement
The authors declare a conflict of interest. M.F. received grants and/or speaker honoraria from MSD, Angelini, Shionogi, Pfizer, Menarini, Gilead and Nordic Pharma. F.M. has participated in advisory boards and/or received speaker honoraria from Angelini, Correvio, Merck Sharp & Dohme (MSD), Nordic Pharma, Pfizer, Astellas, Gilead, Bristol-Myers Squibb (BMS), Janssen, ViiV, bioMérieux, Biotest, Becton Dickinson, Pfizer, and Shionogi. Declared conflicts of interest are outside the submitted work and did not affect the scientific objectivity of this study. The other authors have none to declare.
Figures
Comment in
-
In a Pinch: Cefiderocol for CRAB Infections.Antimicrob Agents Chemother. 2022 May 17;66(5):e0006522. doi: 10.1128/aac.00065-22. Epub 2022 Apr 11. Antimicrob Agents Chemother. 2022. PMID: 35400199 Free PMC article.
-
Cefiderocol and the Need for Higher-Quality Evidence: Methods Matter for Patients.Antimicrob Agents Chemother. 2022 Aug 16;66(8):e0076622. doi: 10.1128/aac.00766-22. Epub 2022 Jul 18. Antimicrob Agents Chemother. 2022. PMID: 35862749 Free PMC article. No abstract available.
-
Reply to Epling and Powers, "Cefiderocol and the Need for Higher-Quality Evidence: Methods Matter for Patients".Antimicrob Agents Chemother. 2022 Aug 16;66(8):e0079522. doi: 10.1128/aac.00795-22. Epub 2022 Jul 18. Antimicrob Agents Chemother. 2022. PMID: 35867525 Free PMC article. No abstract available.
References
-
- World Health Organization. 2021. WHO publishes list of bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-....
-
- Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. 2018. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400. 10.1016/S1473-3099(18)30099-9. - DOI - PubMed
-
- Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, Kokturk F, Ornek T, Celebi G. 2013. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect 141:1214–1222. 10.1017/S095026881200194X. - DOI - PMC - PubMed
-
- Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, Pistello M, Guarracino F, Ghiadoni L, Forfori F, Barnini S, Menichetti F, O Degl’Innocenti SA, Barbieri G, Biancalana M, Borselli M, Nencini E, Spinelli S, Antognoli R, Calsolario V, Monzani F, Paterni S, Baldassarri R, Bertini P, Brizzi G, Rocca AD, Malacarne P, Monfroni M, Piagnani C, Carpenè N, Carrozzi L, Celi A, Desideri M, Gherardi M, Serradori M, Cinotti F, Cipriano A, Park N, Forotti G, Mengozzi A, Masi S, Ruberti F, Sciuto M, Virdis A, Maggi F, Galfo V, Pisa COVID-19 Study Group. 2021. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother 76:1078–1084. 10.1093/jac/dkaa530. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

